August 2025
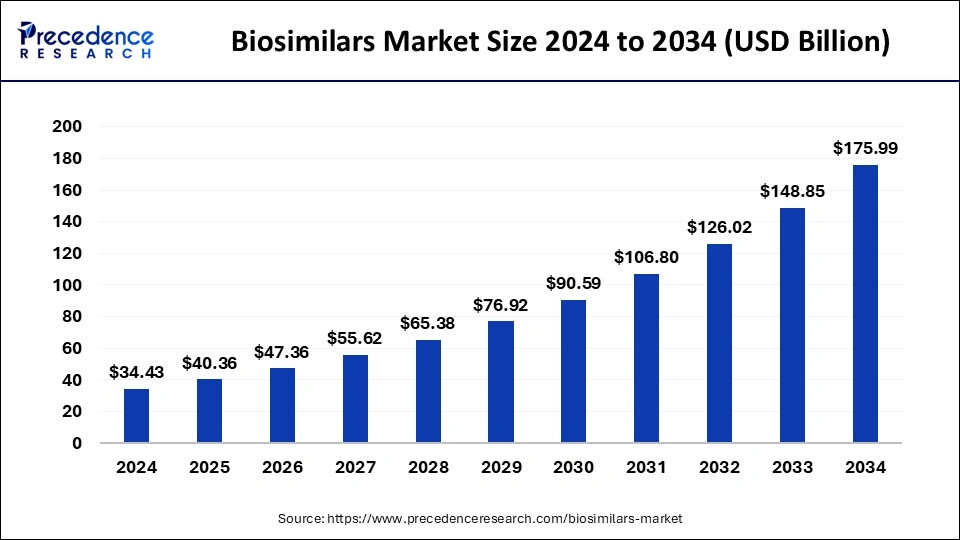
The global biosimilars market size accounted for USD 34.43 billion in 2024 and is predicted to increase from USD 40.36 billion in 2025 to approximately USD 175.99 billion by 2034, expanding at a CAGR of 17.78% from 2025 to 2034. The key players operating in the market are focused on adopting inorganic growth strategies like acquisition and merger to develop advanced technology for manufacturing biosimilars.
Artificial intelligence models like AlphaFold can predict protein structures with high accuracy, expediting the development of biosimilars by ensuring molecular similarity to reference biologics. AI-driven simulations can replace some physical experiments, speeding up the preclinical phase and reducing reliance on expensive lab testing.
AI algorithms can analyze real-time data to optimize production processes, ensuring consistent quality and yield in cell culture and purification stages. Machine learning models can identify potential equipment failures, minimizing downtime and maintaining production schedules. AI can detect deviations in production and identify quality issues early, reducing the risk of batch failures. Advanced analytics can ensure compliance with stringent regulatory requirements by continuously monitoring product consistency.
AI tools can streamline the generation of regulatory submissions by extracting and organizing relevant data. Predictive analytics can manage supply chain logistics, ensuring timely availability of raw materials and distribution of finished products.
The Europe biosimilars market size was exhibited at USD 13.16 billion in 2024 and is projected to be worth around USD 64.82 billion by 2034, growing at a CAGR of 17.34% from 2025 to 2034.
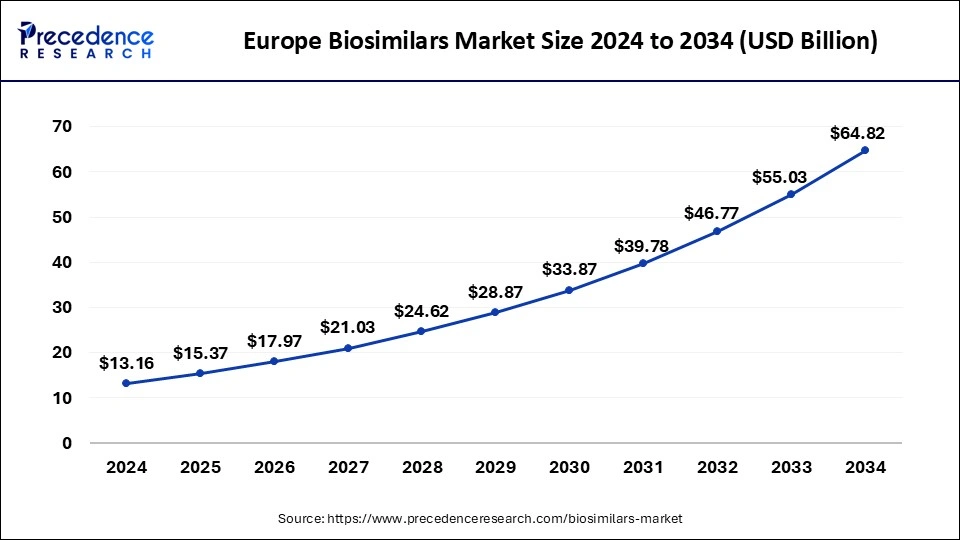
Europe dominated the global biosimilars market in 2024, in terms of revenue, and is estimated to sustain its dominance during the forecast period. The increased adoption of the biosimilars in Europe has boosted the market growth. Moreover, the regulatory authorities have played a crucial role in the adoption of biosimilars by making appropriate and favorable changes towards the approval of biosimilar drugs.
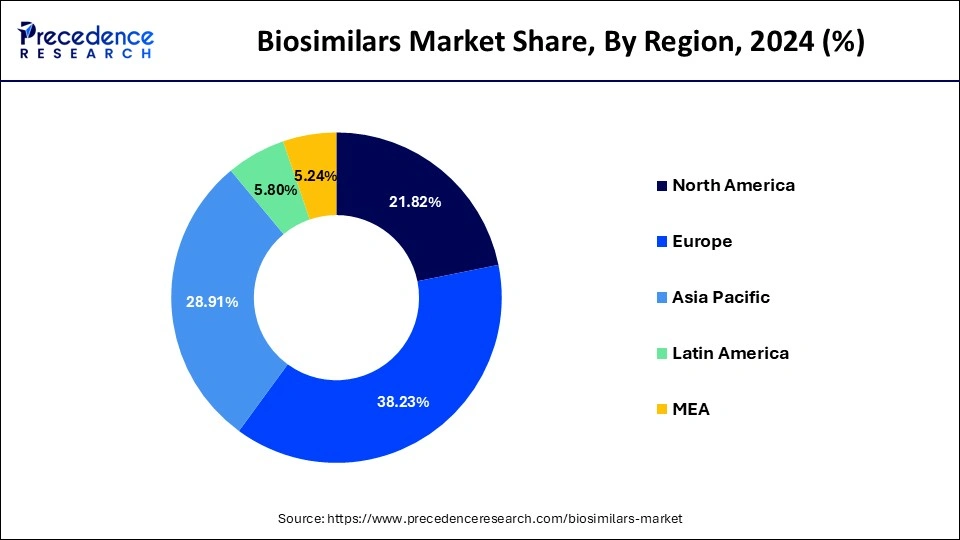
On the other hand, North America is estimated to witness a significant growth rate during the forecast period. The growing popularity of the biosimilars in North America and the presence of numerous market players in the region are investing heavily in the research of the biosimilars, which arew expected to drive the growth of the market in this region. The FDA in US approved around 20 biosimilars in the year 2020. In 2020, Pfizer obtained the FDA approval for its Nyvepria, which is used for lowering the infection incidences. All these factors are anticipated to drive the growth of the biosimilars market in North America, during the forecast period.
The U.S. biosimilars market is significantly expanding, and one of its examples is that, till the date August 2024, nearly 10 different molecules have their biosimilars, and 43 biosimilars have been launched in the U.S., and the U.S. market has witnessed nearly USD 7 billion annual revenue from biosimilars manufacturing. Supportive regulations have also played a key role in the market's expansion in the U.S. For instance, in June 2024, the FDA proposed a guideline about biosimilars that expects an interchangeable designation will no longer require conducting to conduct switching studies as was recommended before.
Market trends in India
The biosimilar market in India is expanding due to many factors, like growing demand for cost-effective treatment solutions and increasing incidences of fatal chronic diseases, which need long-term treatment. Biosimilars provide a more reliable and reasonable option for their related biologics, promoting patients' options for critical healthcare treatments. Also, regulatory support plays a key role in market expansion. The accelerated adoption of biosimilars as a cost-effective solution positions them as a strategic response to the increasing prevalence of chronic diseases resulting from a significant demographic shift. This is achievable by advanced research in biotechnology and increased manufacturing capabilities in India.
Biosimilars are biologic medical products that are highly similar to an already approved original ("reference") biologic drug. They are designed to have the same safety, efficacy, and quality as the reference product, but they are not identical due to the complexity of biologics, which are made from living organisms.
Biosimilars closely resemble the reference biologic in terms of function, structure, and clinical performance. They must demonstrate no clinically meaningful differences in terms of effectiveness and safety compared to the original drug. Typically, less expensive than the reference biologic, making them more accessible to patients. Subject to rigorous testing and evaluation by regulatory agencies like the FDA or EMA to ensure they meet the required standards.
Biosimilars are utilized to treat various conditions, such as cancers, autoimmune disorders, and chronic diseases like diabetes. Examples include biosimilar versions of insulin, monoclonal antibodies, and growth factors.
| Report Highlights | Details |
| Market Size in 2025 | USD 40.36 Billion |
| Market Size by 2034 | USD 175.99 Billion |
| Growth Rate from 2025 to 2034 | CAGR 17.78% |
| Largest Market | Europe |
| Fastest Growing Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product, Application, Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Key Players: Sandoz (a Novartis division), Pfizer, Viatris (formerly Mylan), Teva Pharmaceutical Industries, Amgen
Key Players: IQVIA, ICON, Parexel
Key Players: Sandoz, Pfizer, Biocon Biologics, Celltrion
Key Players: West Pharmaceutical Services, Gerresheimer AG, Berry Global, AptarGroup
Key Players: McKesson Corporation, AmerisourceBergen, Cardinal Health
Key Players: Amgen SupportPlus, Sandoz One Source, Pfizer Oncology Together
Recent biosimilar product approvals in many countries:
Biosimilars and generics are both versions of previously FDA-approved medications and may offer more affordable treatment options to patients. A biosimilar is a biologic medicine that is approved based on a demonstration that it is highly similar to an FDA-approved biological medicine. These therapies are a commonsense solution to rising prescription drug costs and a lower-cost alternative to the most expensive drugs in our healthcare system. The primary benefit of biosimilars is their cost-effectiveness, with drug prices generally about a third lower than the brand-name biologic products.
Regulatory uncertainties:
The regulatory expectations are that the biosimilar manufacturer applies contemporary technologies in their product development and must also adhere to current industry standards and regulatory expectations, which may also have evolved from the time that the reference product was developed and approved. Regulatory needs for biosimilars include rigorous, comprehensive, and evolving. Demonstrating bio-similarity needs rigorous evolution of the proposed biosimilar, including side-by-side comparison with the reference product. As compared to traditional generic drugs, biosimilars must navigate a particularly complex approval process that needs substantial resources, expertise, and time.
Technological advancements in bioprocessing:
Bioprocess technology is a vital part of biotechnology that deals with processes combining the complete living matter or its components with nutrients to make specialty chemicals, reagents, and biotherapeutics. The processes form the backbone of translating discoveries of life sciences into useful industrial products. The major benefits of bioprocesses over traditional chemical processes are that they require mild reaction conditions, are more specific and efficient, and produce renewable by-products. The development of recombinant DNA technology expanded and extended the potential of bioprocesses. Implementing robotic technology into the manufacturing process translates to savings on labor costs, which increases productivity. It enables manufacturers to grow without paying additional salaries.
The monoclonal antibodies segment held a dominant presence in the biosimilars market in 2024. Monoclonal antibodies will constitute the largest group for biosimilars in 2024 because of their long-term use in chronic diseases like cancer, autoimmune disorders, and inflammatory disorders. The expiry dates of the patents belonging to many first-generation blockbuster biologics opened up the entry for their biosimilar versions in the market, ensuring that the product could be offered at a cheaper rate and hence enjoy wider market reach.
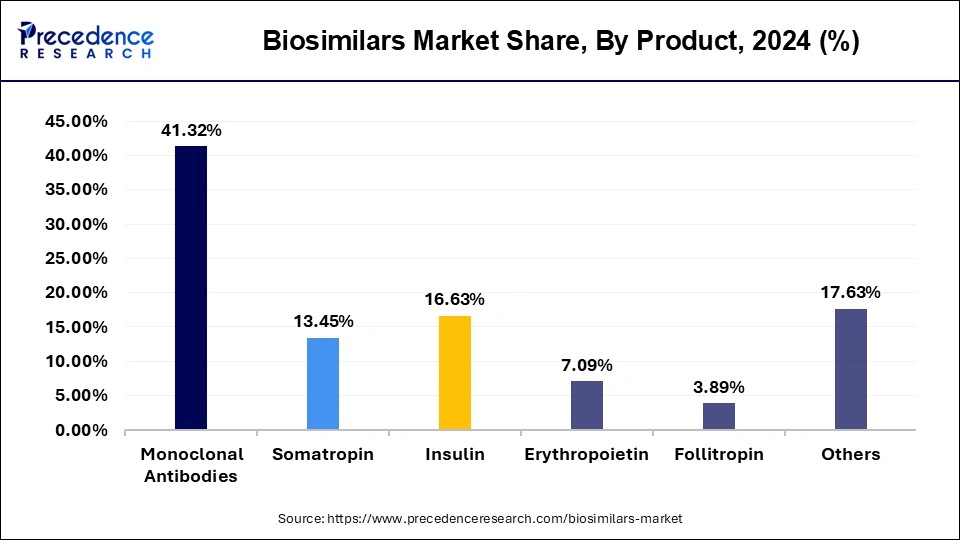
The erythropoietin segment is expected to grow at the fastest rate in the market during the forecast period of 2025 to 2034, owing to the increasing prevalence of anemia in chronic kidney disease, cancer treatment, and other indications. Market growth would further be supported by mounting arrears for biosimilar erythropoietin adoption and increasing demands for targeted therapy and supportive treatment, rendering it the major plight for manufacturers throughout the coming decade.
The oncology segment accounted for a considerable share of the biosimilars market in 2024. In 2024 registered a sizeable share because of oncology, with cancer seeing highest prevalence and demand for cheaper biologics. Oncology biosimilars, mainly monoclonal antibodies and supportive therapies, have tremendously helped in offering life-saving medicines to more patients and alleviating the costs borne by healthcare systems for the said medicines. This promise, owing to R&D activity and favorable regulatory frameworks in the major market regions, shall eventually seep out to the segment.
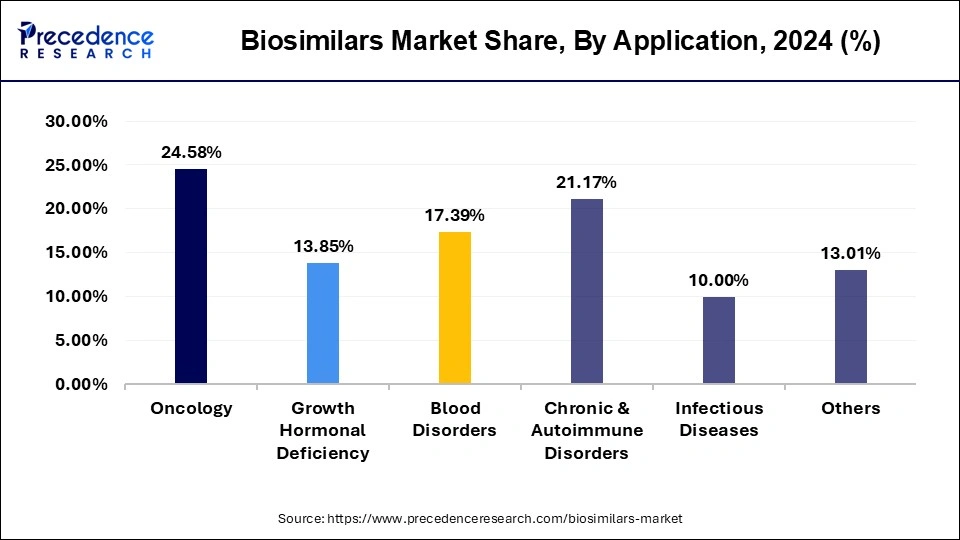
On the other hand, the growth hormonal deficiency segment is expected to be the fastest-growing segment during forecast period. The rising chances of kids getting affected with rare growth hormone deficiency during pregnancy is expected to drive the market. According to the National Organization for Rare Disorders, there are 50% chances of getting growth hormone deficiency to the child during pregnancy.
The growth hormonal efficiency segment is projected to experience the highest growth rate in the market between 2025 to 2034 due to growth hormone deficiency. Such a growing rate is contributed to by rising diagnosis-to-lowers treatment rates for both pediatric and adult patients, advances in recombinant DNA technologies, and the availability of biosimilar growth hormones. Greater awareness and clamping down on high costs will, therefore, drive adoption in this segment, making it one of the major growth sectors in the biosimilars market.
Biosimilars Market Revenue, by Application, 2022-2024 (USD Million)
| Application | 2022 | 2023 | 2024 |
| Oncology | 6,102.66 | 7,183.57 | 8,463.88 |
| Growth Hormonal Deficiency | 3,471.72 | 4,066.32 | 4,767.24 |
| Blood Disorders | 4,386.88 | 5,122.82 | 5,987.84 |
| Chronic & Autoimmune Disorders | 5,287.81 | 6,205.83 | 7,290.07 |
| Infectious Diseases | 2,537.22 | 2,953.96 | 3,442.38 |
| Others | 3,339.07 | 3,866.68 | 4,480.91 |
The contract research and manufacturing services segment led the market. The CRAMS segment held dominance in the biosimilars market in the year 2024, being an end-to-end solution provider for development up to production and regulatory compliance. Most pharmaceutical companies tend to outsource to CRAMS providers in order to save capital investment, speed up launch schedules, and assure quality compliance at the world markets. With increasing difficulties in biologics manufacturing, this segment is further gaining dominance.
The in-house segment is set to experience the fastest rate of market growth from 2025 to 2034. As the number of big biopharma players investing in the development of in-house manufacturing capabilities grows, these players want to retain complete control over quality, intellectual property, and supply-chain operations. Increasing profit margins with the ability to scale production quickly in response to market demand or regulatory changes is another explanation} behind this trend.
Biosimilars Market Revenue, by Manufacturer, 2022-2024 (USD Million)
| Manufacturer | 2022 | 2023 | 2024 |
| Contract Research and Manufacturing Services | 3,842.00 | 4,509.02 | 5,296.80 |
| In-House | 21,283.36 | 24,890.17 | 29,135.53 |
The market is moderately fragmented with the presence of several local companies. These market players are striving to gain higher market share by adopting strategies, such as investments, partnerships, and acquisitions & mergers. Companies are also spending on the development of improved products. Moreover, they are also focusing on maintaining competitive pricing.
The various developmental strategies like acquisition, partnerships, mergers, and government policies fosters market growth and offers lucrative growth opportunities to the market players.
By Product
By Application
By Manufacturer
By Region
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
August 2025
April 2025
August 2025
August 2024