February 2025
The global eClinical solutions market size is calculated at USD 12.82 billion in 2025 and is forecasted to reach around USD 25.85 billion by 2034, accelerating at a CAGR of 8.10% from 2025 to 2034. The North America eClinical solutions market size surpassed USD 5.46 billion in 2024 and is expanding at a CAGR of 7.60% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global eClinical solutions market size was estimated at USD 11.38 billion in 2024 and is anticipated to reach around USD 25.85 billion by 2034, expanding at a CAGR of 8.10% between 2025 and 2034.
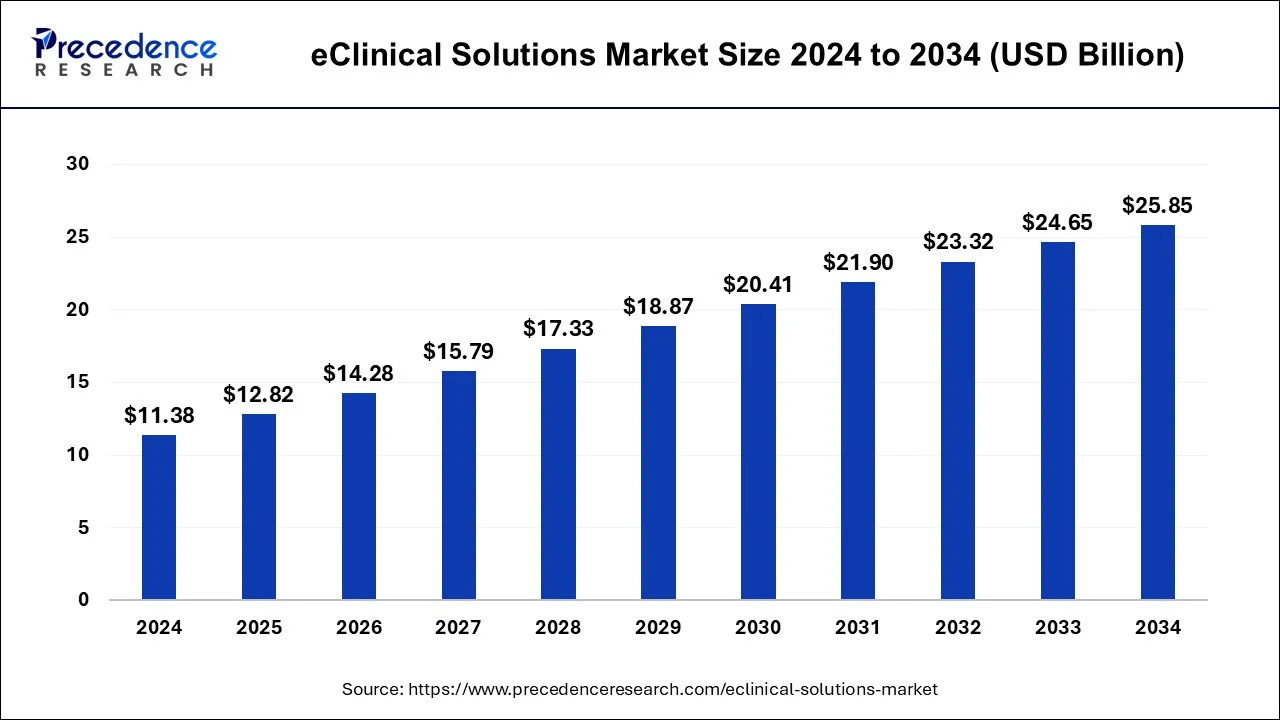
The U.S. eClinical solutions market size accounted for USD 4.46 billion in 2024 and is expected to be worth around USD 9.64 billion by 2034, poised to grow at a CAGR of 8.94% from 2025 to 2034.
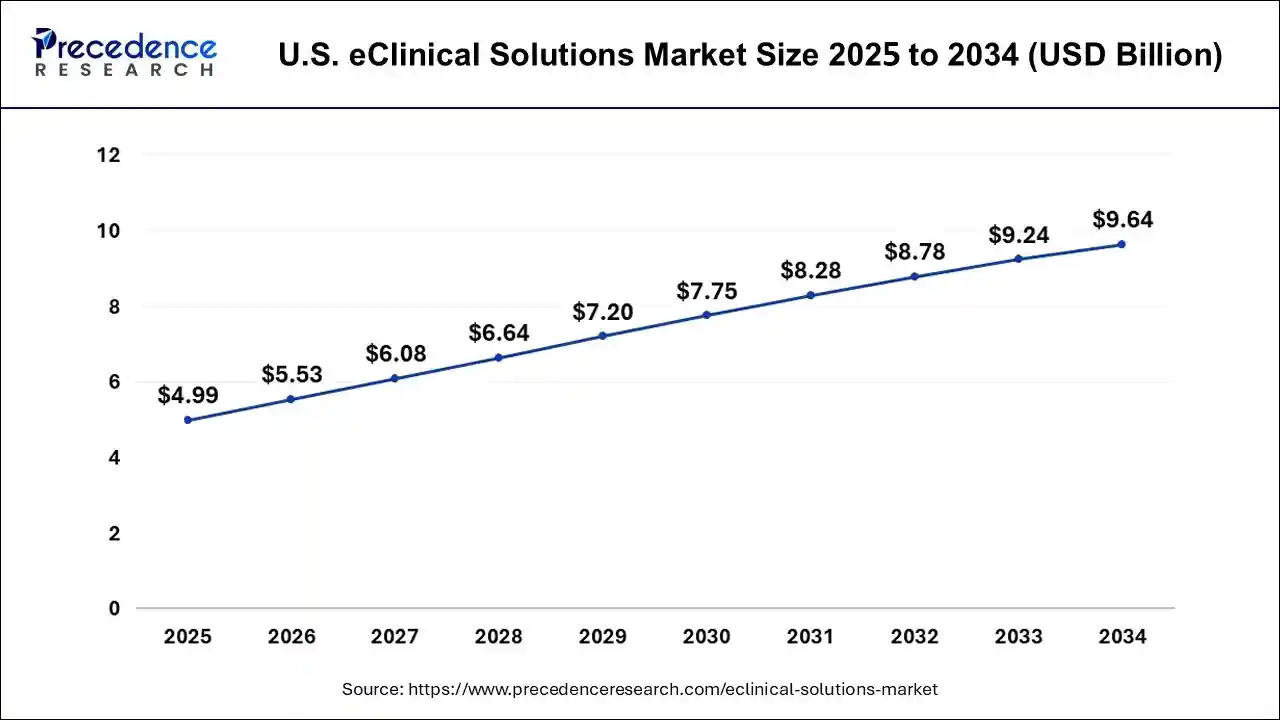
North America led the global eClinical solutions market in terms of revenue in the year 2024. Rising prevalence of lifestyle accompanied with several diseases such as cardiac disorder and diabetes along with increasing number of target population are the key factors that poised to stimulate the demand of eClinical solutions in the region. Apart from this, the Asia Pacific exhibits lucrative growth over the analysis period owing to rising prevalence of chronic diseases that include cardiovascular conditions, cancer, and infectious diseases. Furthermore, many Asian countries such as India, China, Japan, and Korea outsources large number of clinical trials due to large patients population in the countries, thereby boosting the adoption of eClinical solutions in above mentioned regions.
Integration of software solutions in clinical trials provides tremendous market growth. Further, rising trend of clinical trial outsourcing services to Contract Research Organizations (CROs) coupled with rising number of life sciences organizations and CROs poised to help the market to gain significant traction over the forecast period. Increasing research activities in Asian countries for the development of cost-effective solutions or modules expected to spur the market growth.
Presence of strict regulations for clinical trial together with rising need for safety monitoring plays a vital role in the fuelling adoption of eClinical solutions especially in the developed countries such as the United States. For instance, the National Institutes of Health and the U.S. Department of Health and Human Services have issued stringent regulations on clinical trial registration requirements; additionally they promote clinical data sharing.
Biopharma and pharma companies seeks surge in demand of software solutions for clinical trials that accounts as one of the primary factor to stimulate the overall market growth. Apart from this, favorable government policies to expand the end-users of eClinical solutions as well as offer grants to substantiate trials are likely to propel the market growth during the analysis period.
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 8.10% |
| Market Size in 2024 | USD 11.38 Billion |
| Market Size by 2034 | USD 25.85 Billion |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | By Product, By Development Phase, By Delivery Mode and By End-use |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Enormous demand for real-time data access
The advancements in the healthcare sector across the globe have forced providers to adopt remote monitoring, real-time data access supports remote monitoring of patients participating in clinical trials. With the use of eClinical solutions, patients can report data remotely through electronic patient-reported outcome tools or wearable devices. Real-time data access enables healthcare professionals, researchers, and sponsors to make informed decisions promptly. With eClinical solutions, data collected during a clinical trial is instantly available, allowing stakeholders to monitor patient safety, track study progress, and make critical decisions in real-time. This facilitates faster intervention, adjustment of trial protocols, and immediate response to safety concerns or adverse events. Considering the factors that offer real-time data with eClinical solutions, the element is observed to act as a driver for the market.
Rising number of clinical trials
With the increasing number of clinical trials, collaboration among various stakeholders becomes crucial. eClinical solutions facilitate seamless communication and collaboration between sponsors, investigators, CROs, regulatory authorities, and other involved parties. These solutions provide centralized platforms for real-time data sharing, document exchange, and remote monitoring. Enhanced collaboration accelerates decision-making, promotes transparency, and ensures effective coordination among trial participants. The growing number of clinical trials generates vast amounts of data that require analysis and reporting. eClinical solutions provide advanced analytics capabilities to extract valuable insights from the collected data. These solutions offer tools for data visualization, statistical analysis, and generation of reports, enabling researchers and trial sponsors to make informed decisions and communicate trial outcomes effectively. Thus, the requirements for data analysis along cost efficiency offered by eClinical solutions for clinical trials makes it a driving factor for the market.
Risk of errors
Errors can occur during the data entry process, leading to inaccuracies and inconsistencies in the data. Human errors, such as typos, incorrect data input, or data mapping mistakes, can negatively impact the integrity and reliability of the collected data. Even small errors in data entry can have significant consequences, affecting the outcomes of clinical trials and potentially compromising patient safety. Moreover, ensuring compliance can be challenging, and non-compliance can lead to penalties, legal issues, or data integrity concerns, posing a restraint to the adoption of eClinical solutions.
Lack of standardization
In clinical trials, data is generated from multiple sources, including different sites, investigators, devices, and systems. Without standardized data models and formats, the process of integrating and harmonizing data becomes complex and time-consuming. This lack of standardization can result in data inconsistencies, errors, and difficulties in performing cross-study analysis, meta-analysis, or comparative effectiveness research. In the absence of standardized data collection methods and formats, there is a higher risk of data quality issues, including errors, inconsistencies, and missing data. This lack of standardization can impact the reliability and integrity of clinical trial data, potentially leading to flawed analysis, biased results, or inaccurate conclusions. Thus, lack of standardization creates a restraint for the market.
Technological advancements
The integration of big data analytics and eClinical solutions allows for in-depth analysis of vast amounts of clinical and non-clinical data. It enables the identification of novel biomarkers, identification of patient subgroups, optimization of trial design, and predictive modeling. These insights can lead to more personalized and targeted interventions, improved trial outcomes, and enhanced patient care. Advanced communication and collaboration tools facilitate seamless interactions among study teams, investigators, and sponsors. Virtual trial platforms enable decentralized or hybrid trial models, eliminating the need for physical site visits and reducing logistical challenges. These technologies enable broader participant recruitment, faster trial execution, and cost savings; all these are observed to offer technological advancements in the market.
Global expansion of clinical services
The use of real-world data in clinical research is expanding, enabling the evaluation of treatment outcomes and safety profiles in real-life settings. The global expansion of clinical services necessitates collaboration among various stakeholders, including pharmaceutical companies, CROs, research institutions, regulatory bodies, and technology providers. eClinical solutions offer platforms and tools that enable seamless collaboration, data sharing, and secure communication among these entities. The opportunity lies in fostering partnerships and alliances to deliver integrated eClinical solutions that meet the specific needs of global clinical trials and research endeavors.
Complexities in adoption in underdeveloped areas
Underdeveloped areas may have limited access to modern technology and digital devices. Clinical research sites, healthcare facilities, and research organizations in these areas may not have the necessary resources to invest in eClinical solutions or update their existing technology infrastructure. The lack of access to computers, tablets, or smartphones can impede the adoption of eClinical solutions, as these tools are essential for data capture, entry, and communication. Thus, complicated adoption in underdeveloped areas is observed to create a challenge for the market.
The electronic data capture segment dominated the market with the largest share in 2024. The strong emphasis on optimizing clinical workflows has boosted the adoption of electronic data capturing (EDC) solutions. These solutions allow quicker and more efficient data entry by reducing human errors. In addition, EDC solutions reduce the time required for data processing and analysis, accelerating clinical trials. The rise in popularity of remote clinical trials further bolsters segmental growth.
The electronic trial management system segment is expected to expand at the fastest rate in the coming years. With the rising popularity of telehealth and remote clinical trials, the need for an electronic trial management system (ETMS) is increasing. It automates several tasks involved in clinical trials, reducing administrative burden. ETMS provides centralized data storage and management, enhancing the security of trial data. Moreover, ETMS leads to significant cost savings, making it an attractive option for clinical trials.
Web-hosted eClinical solutions captured maximum market value share in 2024. The dominance of the segment is mainly due to its associated benefits that include usability, easy accessibility, and lower investments required. Further, the web-hosted solutions can be easily customized because of which providers can customize their offering as per the user group. Further, these solutions have higher level of interoperability. The aforementioned factors help the segment to maintain its position during the forecast period.
However, Cloud-based solutions predicted to exhibit strong growth rate during the analysis period due to integrated features such as negligible handling costs, high accessibility, flexibility, and easy data backup. Real-time data obtained through these systems that enable users to make quick decisions along with it also provides high-quality information for risk monitoring. The above associated advantages projected to bolster the demand for cloud-based solutions during the forecast period.
eClinical Solutions Market Revenue (USD Million), By Delivery Mode, 2022-2024
| Delivery Mode | 2022 | 2023 | 2024 |
| Licensed enterprise (on-premise) Solutions | 1,060.8 | 1,206.7 | 721.1 |
| Cloud-based (SAAS) Soutions | 3,211.6 | 3,698.7 | 2,213.9 |
| Web-hosted (On-Demand) Solutions | 4,457.7 | 5,098.9 | 2,993.3 |
The CROs dominated the global eClinical solutions market in 2024 and anticipated to witness remarkable growth in the coming years. This is attributed to the rising concern of pharmaceutical companies to reduce their overall expenditure. Moreover, increasing application of eClinical solutions in research activities has further broadened the scope of the segment. Benefits of outsourcing clinical trials to CROs comprise enhanced productivity, increased efficiency of services, cost advantages, and increased focus on core areas of development that are critical for a company’s growth.
On the other hand, pharmaceutical and biotechnology companies expected to witness prominent growth over the forecast period. The eClinical solutions not only enhances the efficiency of the trial but also reduce the time and cost required during a drug development.
eClinical Solutions Market Revenue (USD Million), By End-use, 2022-2024
| End-use | 2022 | 2023 | 2024 |
| Contract Research Organizations (CROs) | 3,446.6 | 3,963.9 | 2,334.7 |
| Medical Device Companies | 1,511.6 | 1,726.0 | 1,030.4 |
| Pharma/Biotech Companies | 2,272.5 | 2,599.5 | 1,526.3 |
| Hospitals & Clinics | 1,030.7 | 1,183.0 | 713.5 |
| Others | 468.8 | 531.9 | 323.3 |
The global eClinical solutions market encounters huge competition among the market players owing to a race among players to capture the untapped market opportunity. These players adopt various growth strategies that are mergers & acquisitions, new product development, and collaborations that help them to consolidate their position in the market. For example, in September 2019, Anju Software Inc. acquired OmniComm Systems to enrich its portfolio of eClinical solutions with innovative data analytical capabilities.
This research study comprises complete assessment of the market by means of far-reaching qualitative and quantitative perceptions, and predictions regarding the market. This report delivers classification of marketplace into impending and niche sectors. Further, this research study calculates market size and its development drift at global, regional, and country from 2020 to 2032. This report contains market breakdown and its revenue estimation by classifying it on the basis of product, development phase, delivery mode, end-use, and region:
By Solution
By Development Phase
By Delivery Mode
By End-Use
By Regional Outlook
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
February 2025
April 2025
January 2025
March 2025