March 2025
The European Medicines Agency has granted its qualification opinion for the first AI Qualification Opinion ever entered over an AI-based diagnostic device, called AIM-NASH. This action aims to support pathologists in evaluating liver biopsies to gauge the severity of metabolic dysfunction-related steatohepatitis, also known as MASH. This disease is progressive and affects the liver. MASH used to be called nonalcoholic steatohepatitis. Fat gets stored, inflamed, and undergoes fibrosis among several possible transformations.
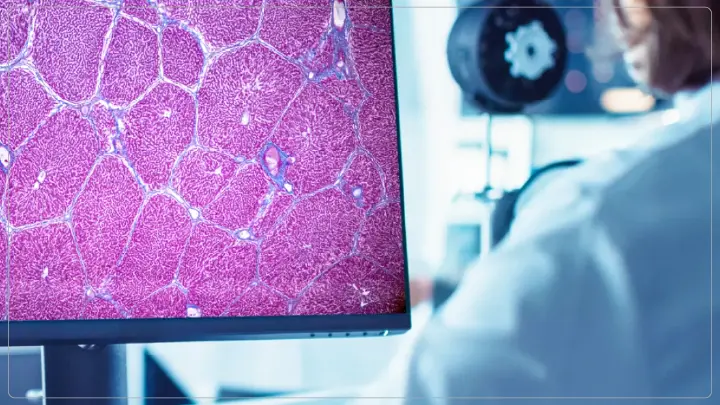
These are often in the presence of other metabolic risk factors including obesity, Type 2 diabetes, hypertension, higher visceral fat, or dyslipidemia. This can lead to advanced liver disease which is probably irreversible unless treated. Early diagnosis is essential for treatment development.
AIM-NASH, an artificial intelligence application, improves the standards and quality of liver biopsy sample evaluation in clinical trials. Due to the next generation of machine learning algorithms used in AIM-NASH, reproducibility in MASH diagnosis is enhanced further, allowing researchers to make more accurate measures of disease severity. Reduced variability, data-based reliability, and speed and credibility in MASH research are some of the key features. AIM-NASH has also received a qualification opinion from the EMA, establishing its scientific validity for future clinical trial applications. The tool improves reproducibility and repeatability in the automated analysis of biopsies; rapid drug development via more accurate clinical trial data; and enhanced patient outcomes through systematic diagnostics.
Recently, it has been recognized that a liver biopsy is the main clinical gold standard for diagnosing MASH, but it is always associated with several important drawbacks like high variability, long turnaround times, and its invasive nature. AIM-NASH gets rid of all these limitations by delivering a more objective and efficient approach, thus eliminating the need for additional biopsies. The AIM-NASH is considered a 'locked' AI model, which means that any modification related to its machine learning algorithm should be done under requalification. There is a suggestion for enhancement from EMA in the future, declaring that each significant update should get through another round of regulatory review to be accepted scientifically; in sync with a multi-year project on how AI could be safely and responsibly integrated into European medicines regulatory network at an operational level.
AIM-NASH is an AI-assisted diagnostic tool for the MASH. The EMA approval of AIM-NASH sets an important precedent in AI-based healthcare. AI might open the door for non-invasive diagnostic alternatives, including AI-assisted imaging, thus lessening dependence on biopsies. Qualification Opinion for AIM-NASH by the EMA sets a new standard for clinical trials that will ensure that potential treatments reach patients with greater efficiency. As AI revolutionizes medicine, entities like EMA must assess and approve these innovations to ensure that they meet all scientific and ethical criteria.
March 2025
March 2025
March 2025
March 2025