January 2025
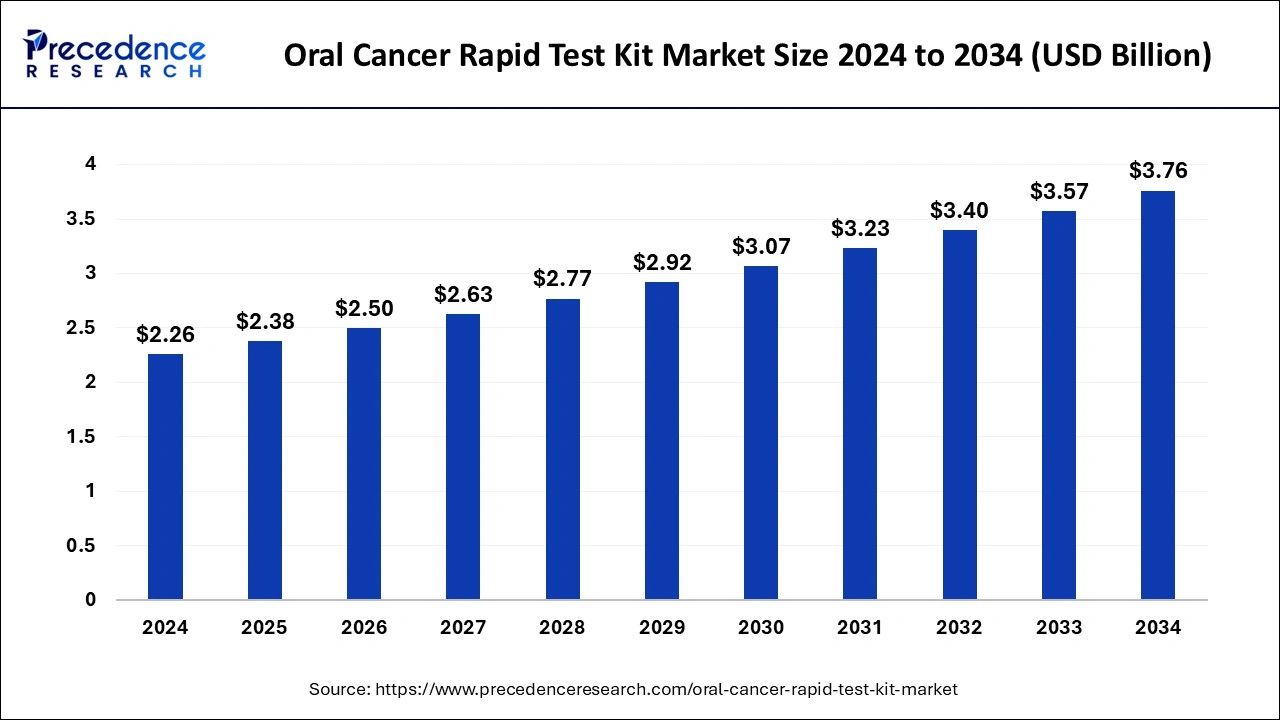
The global oral cancer rapid test kit market size is calculated at USD 2.38 billion in 2025 and is forecasted to reach around USD 3.76 billion by 2034, accelerating at a CAGR of 5.21% from 2025 to 2034.
The global oral cancer rapid test kit market size was accounted for USD 2.26 billion in 2024 and is expected to exceed around USD 3.76 billion by 2034, growing at a CAGR of 5.21% from 2025 to 2034. The increased public awareness for early detection of oral cancer is the key factor driving the growth of the global oral cancer rapid test kit market. The easy accessibility of kits in retail stores and online platforms is expanding the market growth.
Artificial intelligence is the crucial technology that helps to provide accurate detection of oral cancers and their early symptoms. The integration of AI in the oral cancer rapid test kit market has improved the precision and accuracy of oral cancer detection. AI helps to detect cancer symptoms and roots within a minimum time, as it has become a time-saving technology tool. The home-based healthcare trend has become an initial and major adopter of the AI-based diagnostic solution to generate better outcomes.
The integration of AI in the oral cancer rapid test kit market represents the emerging steps for early, accurate detection and efficient management of oral cancer. The leverage of AI has made it possible for the development of noninvasive diagnostic solutions, which are encouraging the adoption of oral cancer rapid test kits as more convenient and user-friendly solutions. Technological advancements are allowing for more convenient, sensible, and selective oral cancer rapid test kits. Including saliva testing, the OncAlert Oral Cancer LAB test, immunoassay-based testing, sensor-based testing, and point-of-care testing solutions
The prevalence of oral cancer has rapidly increased in recent years across the world due to several factors, including the use of smokeless tobacco, areca nut, and an aging population. Furthermore, the rise in the elderly population has contributed to the range of oral cancers. Elderly patients are continuously seeking convenient, user-friendly diagnostic solutions. The oral cancer rapid test kit market provides easy-to-handle, affordable, accessible, and quick yet accurate results.
The government's focus and initiatives to promote awareness of oral cancer are enhancing public knowledge and addressing the need for early detection of cancer. Additionally, government funding in the oral cancer rapid test kit market and investments in advancing healthcare are making it easy to adopt cutting-edge solutions, including cancer rapid test kits.
Rising collaboration between academic and research institutes is opening novel doors for the development of innovative technologies. Expanding healthcare infrastructure is providing accessibility to advanced and convenient diagnostic solutions. Additionally, the growing trend of home healthcare has increased the adoption of testing kits due to their easy accessibility in retail and online platforms and their easy handling and sensitive abilities.
| Report Coverage | Details |
| Market Size by 2024 | USD 2.26 Billion |
| Market Size in 2025 | USD 2.38 Billion |
| Market Size in 2034 | USD 3.76 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 5.21% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product, End-user, Principle, and Regions. |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa. |
Technology advancements
Advancements in technology are key drivers of the global oral cancer rapid test kit market. Technology advancements such as point-of-care testing systems, lab-on-chip technology, immunoassay-based tests, and biomarker-based testing are leveraging the novel approach of more accurate, reliable, quicker, and user-friendly oral cancer rapid test kits. Additionally, developments of non-invasive diagnostic kits are improving lab settings, which helps to reduce expenses and time consumption. Such technological developments are improving the accuracy and speed of rapid test kits, making them more preferred by healthcare professionals and patients. Technological advancements also allow patients to use self-use kitskits.
Lack of awareness
The lack of awareness of oral cancer rapid test kits among healthcare professionals and patients is the major restraint of the oral cancer rapid test kit market. Despite the rising prevalence of oral cancer and its risk factors, there is insufficient acknowledgment of test kits among people. The limited priority of oral cancer compared to other types of cancers is the key hamper of this knowledge and awareness. The limited understanding of the importance of early detection and the lack of healthcare provider education hampers the adoption of test kits. Launching public awareness campaigns and education programs can help overcome such market challenges.
Home-based testing
The rising prevalence of oral cancer has driven a surge in early diagnostic and convenient solutions. The rising trend toward home healthcare is holding the oral cancer rapid test kit market potential. Patients are seeking convenient, affordable, user-friendly, and accessible healthcare solutions. The kits are easy to handle and are cost-effective solutions for the detection of oral cancer. Additionally, the advancements in technologies are allowing for improved accuracy and user-friendly home-based testing solutions like oral cancer rapid test kits.
Oral cancer is majorly witnessed in the aging population; the demand for home healthcare is high among elderly patients for their comfort and convenience. The easy availability of such kits on the internet is providing more access to cutting-edge technologies. With growing awareness and screening, the demand for oral cancer rapid test kits has increased in home-based diagnostic solutions, which further highlights potential essential advancements in the solution.
The OncAlert oral cancer lab segment led the oral cancer rapid test kit market in 2024. The segment growth is attributed to the non-invasive properties and user-friendly nature of the OncoAlert oral cancer test. OncAlert oral cancer lab tests provide an accurate understanding of the process of oral cancers and their possible diagnostics to physicians. Their reliability and accuracy have made them majorly preferred by healthcare professionals. Additionally, the proven effectiveness of OncAlert oral cancer lab tests in clinical settings is the key reason behind the segment expansion.
The immunoassay segment dominated the oral cancer rapid test kit market in 2024. The immunoassay tests are highly sensitive and specific in nature for detecting oral cancer biomarkers. The well-established immunoassay technology is widely used in clinical diagnostics. Additionally, the ability to detect a wide range of biomarkers is making them a priority to utilize for oral cancers. The segment growth is associated with the sensitive nature and rapid and precision result abilities of immunoassay-associated test kits.
The hospitals segment and the diagnostic center segment dominated the oral cancer rapid test kit market in 2024 due to the large number of patients in hospitals and diagnostic centers. The advancements of hospitals and diagnostic centers are making easy access to personalized oral cancer rapid test kits. Moreover, the availability of trained healthcare professionals to administer and interpret the outcomes of oral cancer rapid taste kits has been responsible for rising hospitalizations and patient enrollments in diagnostic centers. Government funding for cancer patients and easy access to advanced technologies in hospitals and diagnostic centers are the major factors driving segment growth.
On the other hand, the consumer segment is expected to witness notable growth in the oral cancer rapid test kit market over the forecast period as the demand for home healthcare and self-testing tools has increased. Companies are rapidly investing in innovations and developments of self-testing kits to provide quicker and more accurate results to comply with the rising prevalence of oral cancers. Additionally, the availability of oral cancer rapid test kits on online platforms and in retail stores.
North America dominated the global oral cancer rapid test kit market in 2024 due to the rising prevalence of cancer and government initiatives and investments in key manufacturing companies. The well-established health infrastructure of North America is providing access to cutting-edge technologies, including oral cancer rapid test kits, for better outcomes for patients. The rising awareness and screening of oral cancer fueling the adoption rate of oral cancer rapid test kits.
The United States is leading the North American oral cancer rapid test kit market due to a high oral cancer screening rate in the country. The rapidly growing elderly population has witnessed a large range of oral cancer. The heavy alcohol consumption and cigarette smoking incidence in the United States are also contributing to oral cancer. The advanced healthcare infrastructure and presence of key companies are making the possible success of oral cancer rapid test kits in the country. Canada is the second-largest country leading the regional market due to increased awareness of kits and screening rates.
Asia Pacific will witness substantial growth in the oral cancer rapid test kit market over the forecast period. Asia has the largest patient pool with a prevalence of oral cancers due to a large number of smokeless tobacco consumers. Furthermore, the expanding healthcare expenditure and investments for the adoption of cutting-edge technologies in healthcare are allowing easy access to rapid test kits for the regional people.
The rising awareness of oral cancers and screening rates is driving the adoption of the oral cancer rapid test kit market in Asia Pacific. Additionally, government funding for healthcare expenditure allows the utilization of such user-friendly, quick, and accurate technologies. Government investments and support from local manufacturers also contribute to market expansion.
The Lancet Oncology analysis states that nearly 9 out of 10, that is, 88% of all oral cancer causes, are caused by smoking keless tobacco and areca nut in South-Central Asia. Countries like India, China, and Japan are leading the regional market due to the rising prevalence of oral cancer, rising awareness, and advancing healthcare expenditure.

By Product
By End-User
By Principle
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
January 2025
January 2025
October 2023
December 2024