March 2024
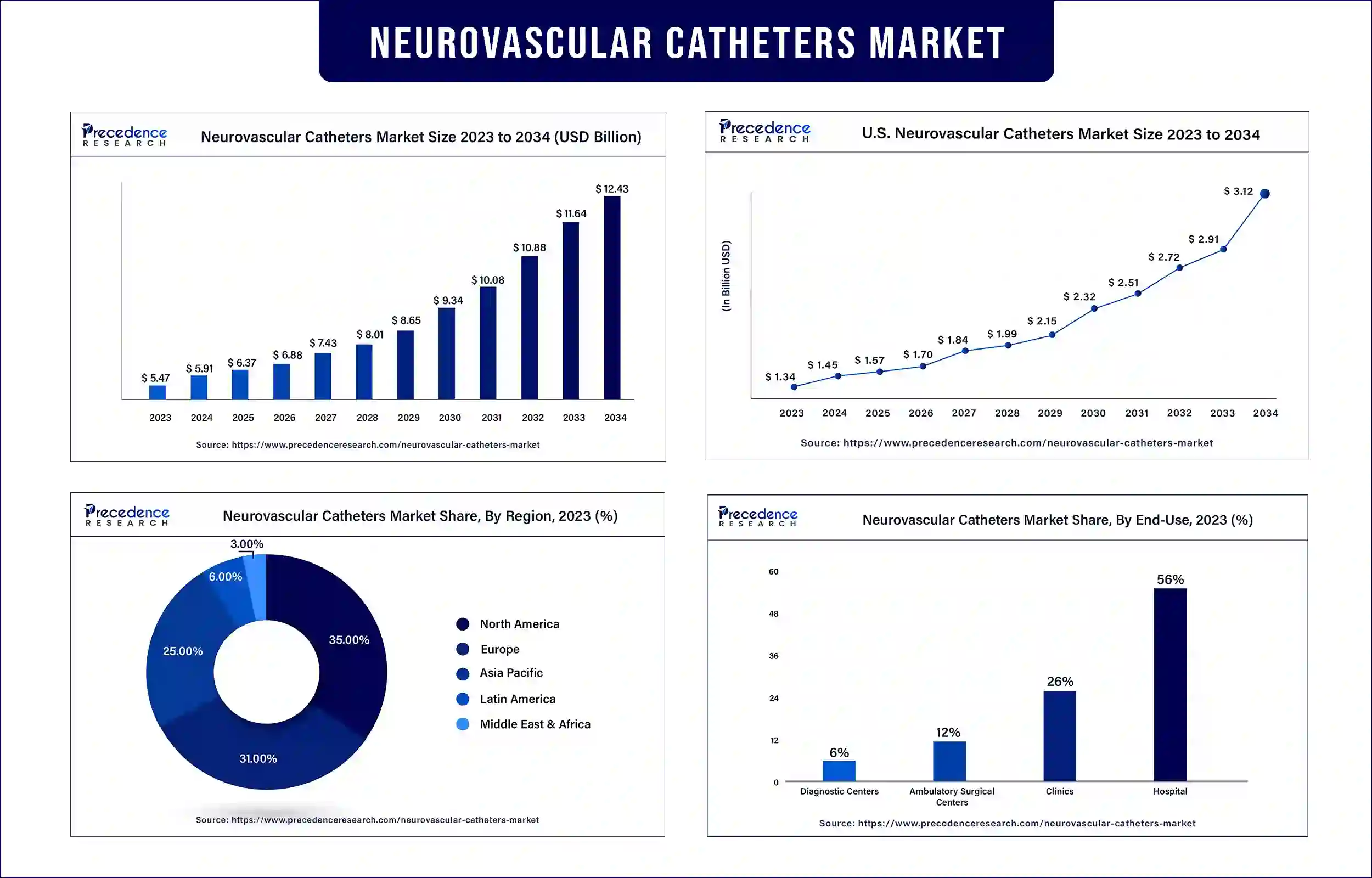
The global neurovascular catheters market surpassed USD 5.47 billion in 2023 and is projected to be worth around USD 11.64 billion by 2033, poised to grow at a CAGR of 7.7% during the forecast period. The rising cases of neurological disorders such as brain stroke and cerebral aneurysms, along with the growing adoption of poor lifestyles are also a major reason for the increase in such disorders and hence neurovascular catheters market.
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/3234
Neurovascular catheters play a vital role in the treatment of neurological disorders it is a tube-like structure that is usually helpful during brain surgeries. Various conditions such as in the case of removal of any blockage from the brain minor endovascular surgery techniques are used to treat the patient. Neurovascular catheters cause less blood loss and help to recover quickly which also increases their preference among all healthcare experts as well as patients. For instance, Scientia Vascular a company that develops and manufactures innovative medical devices and also therapies for neurovascular bases, announced the approval of the FDA for two neurovascular catheters.
One is named Plato 17 microcatheters that offer experts the capability to regulate and steady neurovascular applications and are also DOMSO compatible. The other one is Socrates 38, which is an aspiration catheter for treating ischemic stroke. The neurovascular catheters market is also growing rapidly due to these kinds of new inventions which reduce the load of the health care experts. Some of the market players are contributing the major part to this market such as Stryker Corporation, Penumbra Inc., Medtronic plc, Johnson & Johnson Services Inc., and many others.
Execution of advanced neurovascular access (ANA) driving the neurovascular catheters market
The adoption of neurovascular catheters supports the treatment of several neurological disorders such as cerebral aneurysms and ischemic stroke. These are extensively used to diagnose these neurological conditions and then to treat them and also prevent to increase in any such conditions. For instance, Bendit Technology is a company that develops and manufactures innovative catheters with the help of a unique bending technique, launched Bendit21 in February 2022. It is a steerable microcatheter which is used to treat symptomatic internal carotid artery aneurysm. Currently, catheters are manufactured with a variety of materials and designed in such a way that they can serve a broad spectrum of purposes.
A neurovascular disorder is a condition that creates a blockage in the supply of blood in the brain and spinal cord. It may create blockage by abnormalities, hardening, or narrowing of blood vessels, the blockage created by an embolism or clot. Neurological conditions are gradually becoming the leading cause of poor health in the majority of people across the world. According to the World Health Organisation, more than 3 billion people around the globe are dealing with neurological disorders. Minimally invasive processes are becoming gradually widespread for the treatment of neurological conditions and neurovascular catheters perform a major role in such processes.
As healthcare providers and patients are becoming more conscious of the assistance of minimally invasive processes, hence the requirement for neurovascular catheters is anticipated to upsurge. The continuous research studies are helping the neurovascular catheters market to grow rapidly and introduce innovations to the market. For instance, Stryker Corporation, which is considered one of the leading companies in the medical technology market, introduced a neurovascular complete care solution that comprises less invasive therapies through innovative ischemic and hemorrhagic stroke services and products.
Neurovascular catheters are used for accessing and navigating through gentle blood vessels present in the brain, which is stimulating and can cause to several difficulties such as vessel tear, bleeding, infection, and clotting. Difficulties linked to using neurovascular catheters can cause unembellished neurological shortfalls or even lead to death and can also expand the duration of hospital stay and charges. Additionally, a high rate of risk co-exists while staying with so many patients together. Lack of professionals to handle also causes problems like to operate the device one needs to be trained enough.
A recent development by iVascular
A recent development by Route 92 Inc.
North American region is dominating the neurovascular catheters market for the predicted period of 2024 to 2033 due to growing concern for neurovascular disorders. There are a variety of neurovascular diseases such as arteriovenous malformation, intracranial aneurysms, Central nervous system cavernous hemangioma, moyamoya disease, carotid artery stenosis, brain hemorrhage, dural arteriovenous fistula, and many more. For instance, according to the American Health Association, the number of death cases in the U.S. due to neurovascular diseases increased by 90% which is from 164,000 to 313,000, and DALY loss increased by 37.3% which is 2.3 to 3.7 million. Several innovations in this field are going on to solve the rising issue of neovascular disease death rates. For instance, in July 2023, Stryker, which is an American medical technology corporation, launched the Q Guidance System along with its Cranial Guidance Software to deliver healthcare experts an intraoperative controlling arrangement and also an image-based scheduling device that supports device settlement and patient framework identification at the time of cranial surgery.
Asia Pacific region has witnessed the fastest-growing neurovascular catheter market due to rising advancements in the healthcare sector and government initiatives toward the diagnosis and treatments of such disorders. With the advancement of neurovascular surgery, the demand for neurovascular catheters is also rising.
The rising collaboration among companies drawing the neurovascular catheters market
With the rising cases of diseases such as dural arteriovenous fistula, vertebral artery dissection, intracranial stenosis, cerebrovascular accident, embolism, vascular malformation, arteriovenous fistula, paraganglioma, and many more the concern towards the treatment of these disease are also increasing. Many companies are collaborating to enhance the research studies in this field to manufacture more advanced technology. The growing number of patients admitted to hospitals for treatments, therapies, and surgeries is predicted to boost the neurovascular catheters market. Therefore, the consequent upsurge in patients worldwide, the introduction of technically cutting-edge products, and suitable compensation strategies are causing a rising request for treatments and hence enlarging the potential of the market.
| Report Attribute | Key Statistics |
| Market Revenue in 2024 | USD 5.91 Billion |
| Market Revenue by 2033 | USD 11.64 Billion |
| CAGR | 7.7% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2023 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Segmentation
By Type
By Application
By End Use
Buy this Research Report@ https://www.precedenceresearch.com/checkout/3234
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308
March 2024
July 2024
May 2024
March 2024