January 2025
Systemic Inflammatory Response Syndrome Treatment Market (By Product Type: Meningitis, Urinary Tract Infection (UTI), Pneumonia, Autoimmune Diseases, and Others; By Application: Hospital & Ambulatory Surgical Centers, Specialty Clinics, and Others) - Global Industry Analysis, Size, Share, Growth, Trends Analysis, Regional Outlook and Forecasts, 2024 - 2033
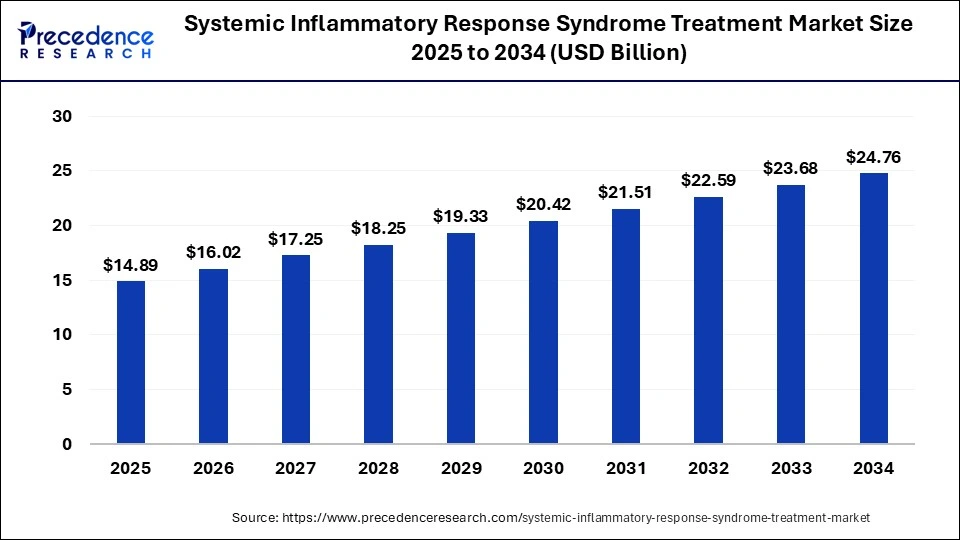
The global systemic inflammatory response syndrome treatment market size was valued at USD 12.90 billion in 2023 and it is projected to hit around USD 23.68 billion by 2033, poised to grow at a CAGR of 6.25% during the forecast period 2024 to 2033. The market growth is driven by an increase in the frequency of systemic inflammatory response syndrome and the prominence of systemic inflammatory response syndrome treatment to remain strong for urinary tract infection treatment.
One of the key factors for assessing post-surgical complications and end-organ dysfunction is systemic inflammatory response syndrome. Syndrome progression is linked to lengthy hospital stays, high incidence of multiple organ dysfunctions, and elevated morbidity. In cardiac surgery, systemic inflammatory response syndrome is among the popular post-operative complications, leading to organ failure or even death. It is expected that the prevalence of systemic inflammatory response syndrome among pediatric patients will increase in the near future. Increasing efforts by the key players to bring effective therapies and drugs for systemic inflammatory response syndrome can create huge growth opportunities in the target industry. As dilemmas about the mass of sepsis increase in systemic inflammatory response syndrome, market players are working to develop effective drugs and therapeutics within the scope of innovation. CytoSorb, a specific extracorporeal cytokine adsorber, is arising as a successful treatment to decrease inflammation and regulate the failure of essential organs such as the lungs, kidneys, heart and brain, amongst many other therapies. Biomarkers for early detection, treatment, and disposal of the condition have also been introduced as demand becomes more prominent for the initial-stage diagnosis of sepsis.
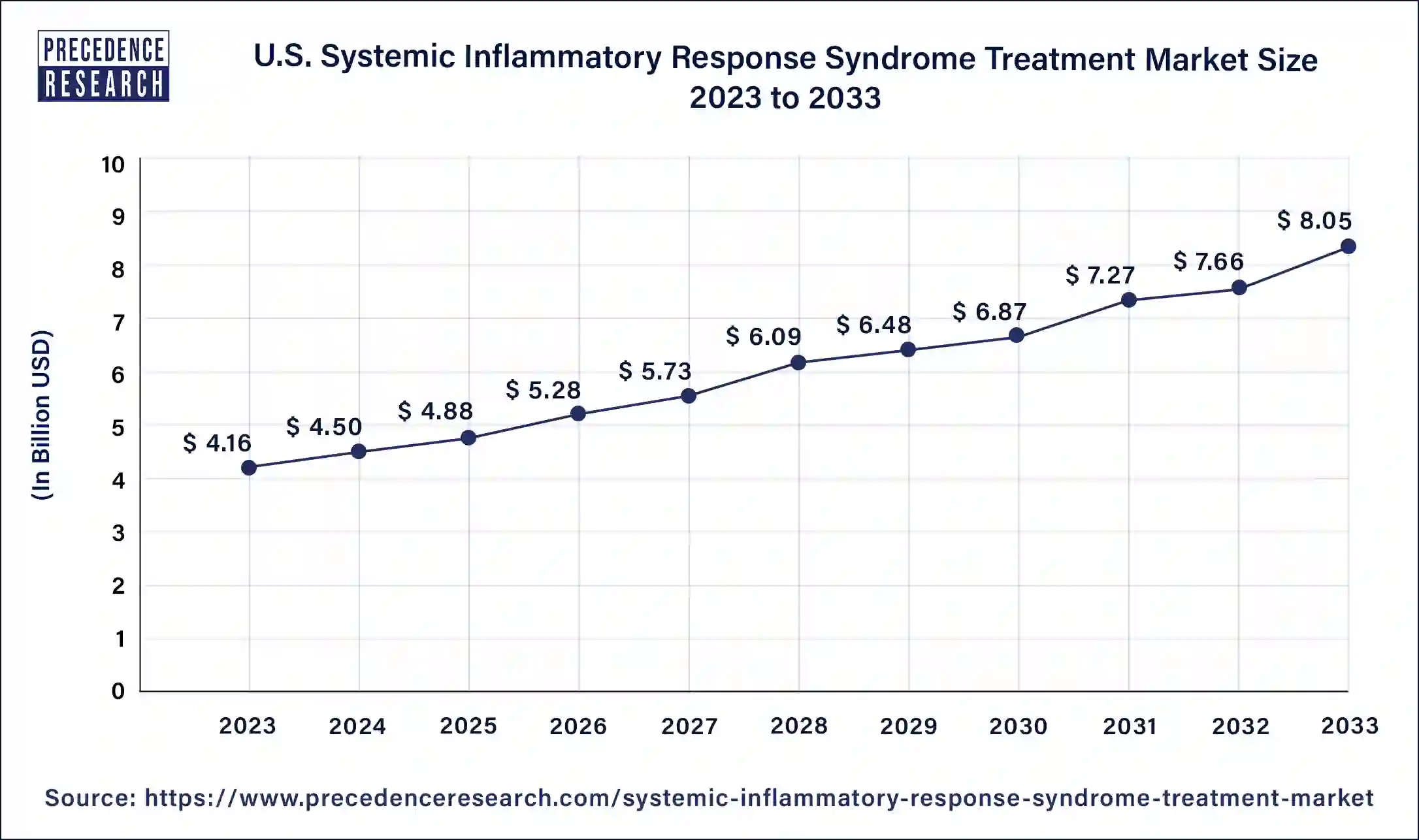
The U.S. systemic inflammatory response syndrome treatment market size was valued at USD 4.16 billion in 2023 and it is projected to hit around USD 8.05 billion by 2033, poised to grow at a CAGR of 6.81% during the forecast period 2024 to 2033.
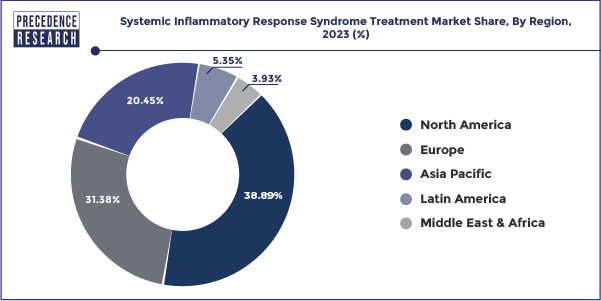
North America held the largest share of 38.89% in 2023 in the systemic inflammatory response syndrome treatment market. The market for Systemic Inflammatory Response Syndrome (SIRS) treatments in North America includes a broad spectrum of therapeutic therapies intended to control the intricate inflammatory response observed in critical illnesses, trauma, and sepsis, among other disorders. Antibiotics for the treatment of sepsis, corticosteroids, immunomodulators, and anti-inflammatory medicines are among the medications in this section that are intended to modulate the inflammatory response. This covers tools like respiration support ventilators, cardiovascular function tracking hemodynamic monitors, and severe organ failure management renal replacement treatment machines. Ongoing research & development initiatives to identify new therapeutic targets, enhance current treatments, and create cutting-edge medical devices define the SIRS therapy market in North America.
Asia pacific is expected to grow at the fastest growth rate during the forecast period. In the Asia Pacific area, the market for treating Systemic Inflammatory Response Syndrome (SIRS) is probably going to be dynamic and changing. Management of SIRS usually entails treating the underlying cause in addition to controlling the systemic inflammatory response. SIRS can result from a variety of underlying diseases, including infections, trauma, burns, or other inflammatory assaults. In SIRS, maintaining appropriate tissue perfusion and avoiding organ failure depend heavily on fluid management. This may entail the use of blood products, colloids, or crystalloids. This would be relevant to the market for intravenous fluids and blood products.
The COVID-19 explosion has had an impact on world's primary markets. COVID-19 effect on the global industry is adverse. The North America is largest systemic inflammatory response syndrome treatment market, but supply and demand for the systemic inflammatory response syndrome treatment has decreased with spread of covid and lockout situations in the U.S. Because of COVID-19, instability of the supply chain, volatility in the supply of raw materials, inadequate manpower in the production facility and less demand for end-use customers have hindered the growth of the target industry in developed and developing economies worldwide.
Leading players of the global systemic inflammatory response syndrome treatment industry are focusing on the strategic partnerships similar acquisitions, mergers in order to enhance their position in the global market and to get the comparative edge. Further companies are launching advanced products in order to fulfill the customized demands of the end-users. These trends are anticipated to continue and will fuel growth of target market over the forecast time-frame. For instance, In June 2020, Asahi Kasei Pharma obtained a new drug approval in the China for agent Flivas in order to treat dysuria related to benign prostatic hyperplasia.
| Report Highlights | Details |
| Market Size in 2024 | USD 13.85 Billion |
| Market Size by 2033 | USD 23.68 Billion |
| Growth Rate from 2024 to 2033 | CAGR of 6.25% |
| Base Year | 2024 |
| Historic Data | 2020 to 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Product, Application |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, Middle East & Africa (MEA) |
| Companies Mentioned | GlaxoSmithKline, AstraZeneca, CytoSorbents Corporation, Cardinal Health, Asahi Kasei, ConvaTec, CHIESI Farmaceutici, Smith & Nephew |
Driver
Personalized Medicine Approaches
Knowing a person's genetic predispositions can assist customize therapy to meet their unique requirements. Genetic testing can detect differences that impact the effectiveness, safety, and metabolism of medications. Personalized medicine relies heavily on biomarkers to help in disease diagnosis, prognosis, and treatment selection. Treatment choices in SIRS can be influenced by biomarkers such as levels of interleukin-6 (IL-6), procalcitonin (PCT), and C-reactive protein (CRP). Utilizing personalized immunotherapy techniques, SIRS is fought by the patient's immune system. Immunomodulatory drugs, like immune checkpoint inhibitors or monoclonal antibodies, can be customized according to the tumor's properties and the patient's immunological profile. Clinicians can minimize systemic side effects while achieving optimal therapeutic concentrations at the site of action by employing targeted drug conjugates or encapsulating pharmaceuticals within nanoparticles.
Restraint
Antibiotic Resistance
One major obstacle to treating systemic inflammatory response syndrome (SIRS) is antibiotic resistance. A dysregulated systemic inflammatory response to a variety of stimuli, including infection, trauma, or surgery, is the hallmark of SIRS, a complex illness. When bacterial infection is suspected or shown to be the underlying cause of SIRS, antibiotics are frequently utilized in its therapy.
Opportunity
Innovative Drug Delivery Systems
Novel drug delivery approaches are transforming the field of systemic inflammatory response syndrome (SIRS) treatment. SIRS carries a considerable morbidity and death rate and is defined by a dysregulated host response to infection or injury. It frequently culminates in multiple organ dysfunction syndrome (MODS). Vasopressors, supportive care, and broad-spectrum antibiotics have been the mainstays of traditional treatment strategies. Using medicine delivery systems based on nanotechnology is one creative strategy. Therapeutic medicines can be specifically delivered to regions of inflammation by using nanoparticles that have been designed to encapsulate them. Drugs are more effectively treated and have less systemic negative effects thanks to this tailored delivery. The creation of drug delivery systems with controlled release is another effective tactic. These systems provide the controlled and continuous release of therapeutic drugs, hence preserving therapeutic concentrations for a prolonged duration.
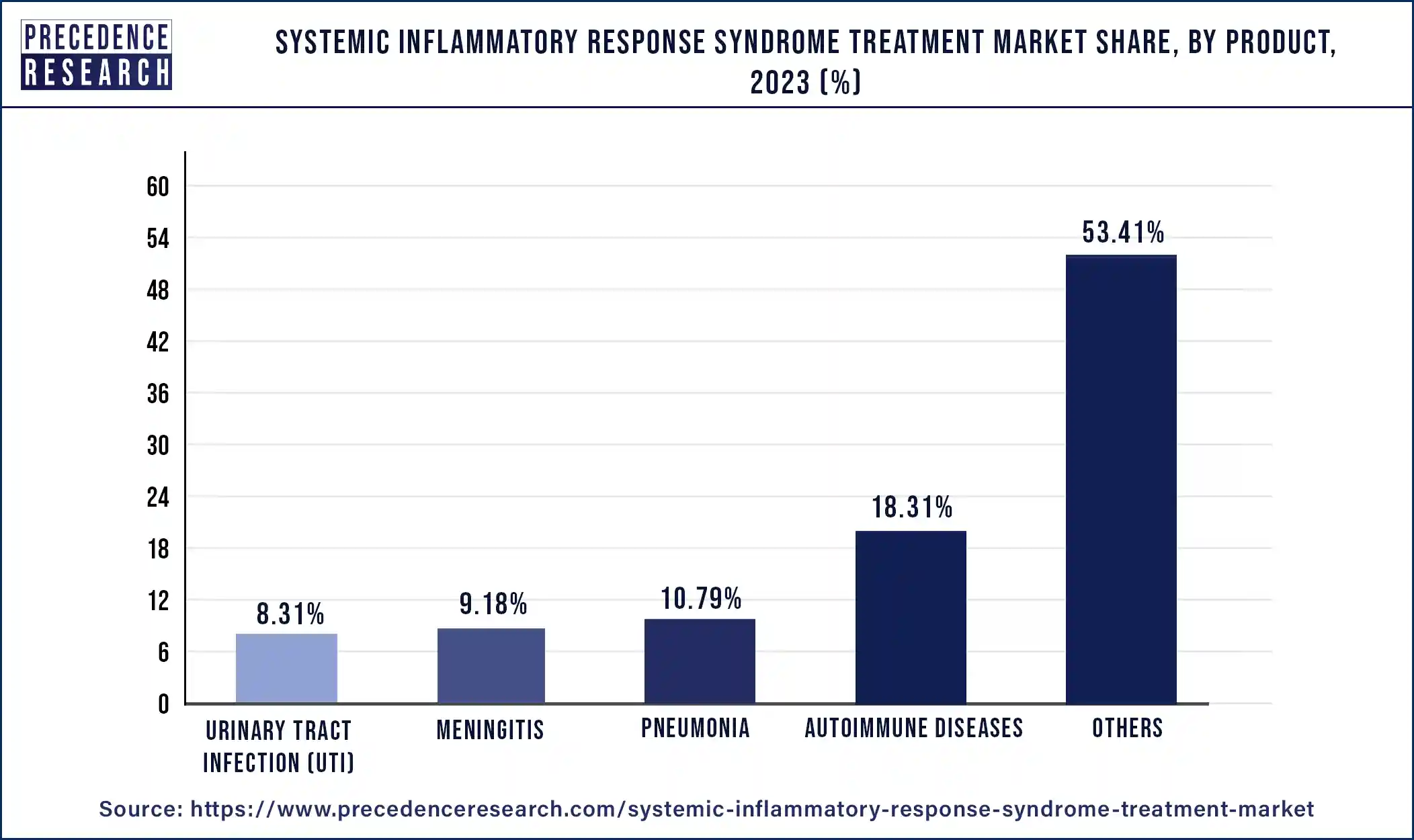
In 2023, the others segment held the largest share of 53.41% in the systemic inflammatory response syndrome treatment market. These treatments, which frequently include corticosteroids, NSAIDs (nonsteroidal anti-inflammatory drugs), and immunomodulatory compounds, aid in reducing inflammation in the body. vital for individuals experiencing severe respiratory distress, which is a frequent SIRS consequence. These tools assist medical professionals in keeping an eye on patients' cardiovascular health, which is essential for controlling shock and organ failure in SIRS. comprising plasma, platelets, and packed red blood cells to treat SIRS-related coagulation issues and anemia. such as procalcitonin (PCT) or C-reactive protein (CRP) testing, which can help diagnose and track the development of SIRS. including ultrasonography, CT scans, and X-rays to detect organ dysfunction and SIRS-related consequences.
Hospital & ambulatory surgical centers segment hold the largest share of 62.15% in systemic inflammatory response syndrome treatment market in 2023. The main location for SIRS patient diagnosis, care, and management is frequently a hospital. Patients with severe conditions, such as those in need of mechanical breathing, critical care, or specialist interventions, receive complete care from them. Hospitals provide a variety of treatments to patients diagnosed with SIRS, including supportive care (oxygen therapy, fluid resuscitation), medication management (vasopressors, antibiotics), and diagnostics (blood tests, imaging scans). Hospital critical care units, including the intensive care unit (ICU), are especially important for treating severe SIRS instances where patients might need life support interventions and close monitoring.
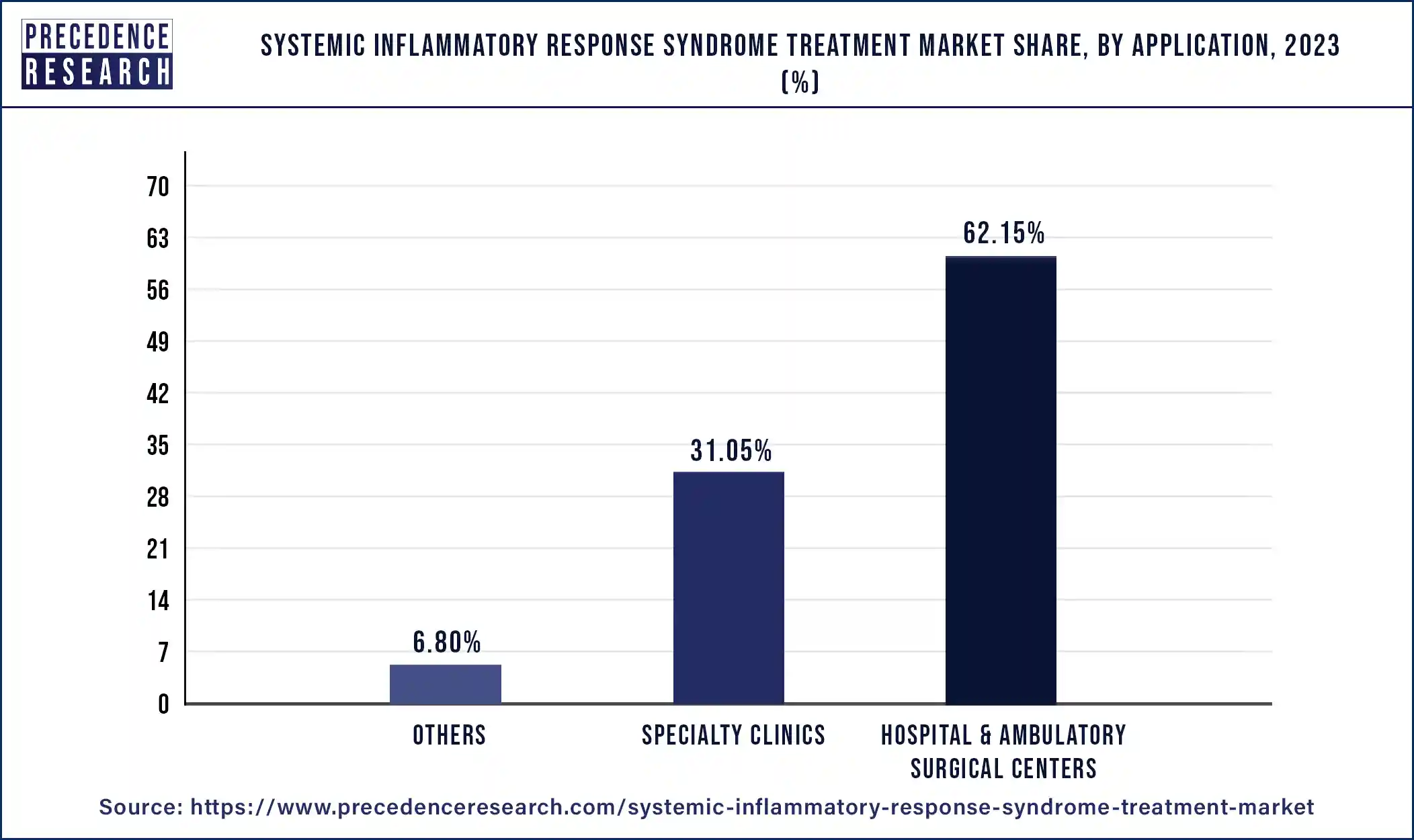
Certain SIRS cases may benefit from the involvement of ASCs in their management, especially if they call for outpatient or surgical procedures. When surgical debridement or infected fluid collection drainage is required, advanced surgical centers (ASCs) with surgical facilities can offer prompt interventions in a more efficient way than standard hospital settings. By offering post-acute care and follow-up services to patients recovering from surgeries or procedures linked to SIRS, ASCs can also function as adjuncts to hospitals. ASCs and hospitals work together as essential components of the healthcare system to manage SIRS, each providing prompt and all-encompassing care to individuals afflicted by the illness.
Major market companies are aiming towards the innovative advancements in order to enhance position in the target industry. Major companies are:
For improved status of systemic inflammatory response syndrome treatment, and strategies accepted by Precedence Research projected the upcoming growth of the systemic inflammatory response syndrome treatment market.
By Product Type
By Application
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
January 2025
July 2024
February 2025
March 2025