February 2024
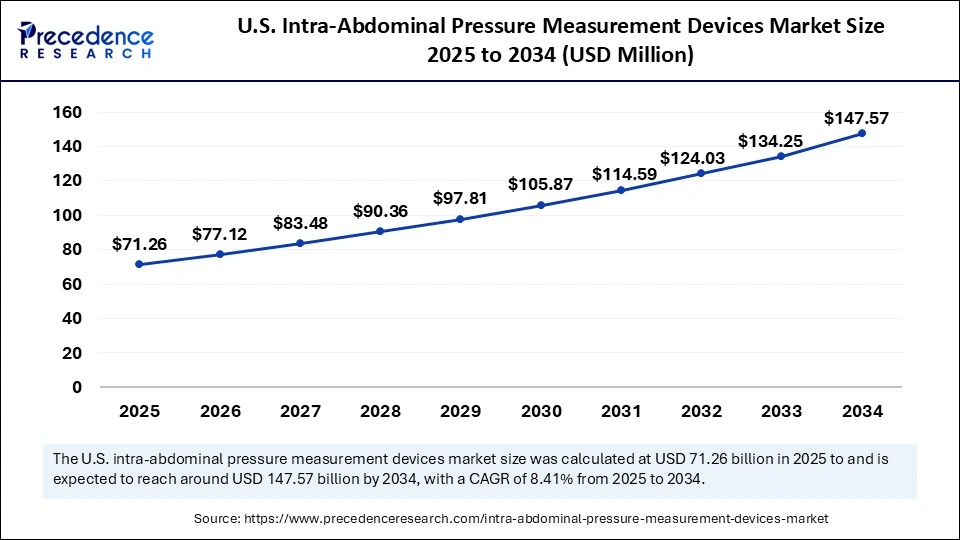
The U.S. intra-abdominal pressure measurement devices market size is calculated at USD 71.26 billion in 2025 and is forecasted to reach around USD 147.57 billion by 2034, accelerating at a CAGR of 8.41% from 2025 to 2034. period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The U.S. intra-abdominal pressure measurement devices market size accounted for USD 65.83 billion in 2024 and is predicted to increase from USD 71.26 billion in 2025 to approximately USD 147.57 billion by 2034, expanding at a CAGR of 8.41% from 2025 to 2034. The market growth is driven by the rising number of trauma incidences, ongoing advancements in medical device technologies and growing number of abdominal procedures in the U.S.
The presence of advanced healthcare infrastructure and major technology providers as well medical device manufacturers in the U.S. is driving the adoption of artificial intelligence and machine learning methodologies in medical applications for enhancing patient safety, treatment outcomes and streamlining workflows for healthcare professionals and medical researchers. Integration of AI in intra-abdominal pressure (IAP) measurement devices can be applied for analysing trends in IAP data with patient parameters enabling detection and proactive intervention of intra-abdominal hypertension (IAH) and abdominal compartment system (ACS) through predictive analytics and early warning systems.
Furthermore, AI can assist in making informed clinical decisions, in development of innovative non-invasive measurement techniques with enhanced reliability and accuracy, in personalizing treatment strategies and also in image analysis for related conditions such as abdominal trauma or appendicitis which use ultrasound imaging.
Intra-abdominal pressure measurement devices are medical instruments applied for monitoring and assessing the abdominal cavity pressure which is necessary for the detection and management of conditions such as abdominal compartment syndrome (ACS) and intra-abdominal hypertension (IAH). These devices are extensively used in various surgical settings, critical care and trauma management.
The rising incidences of trauma cases and critical illnesses, increased number of surgical procedures, growing awareness about IAH in medical professionals, ongoing research activities for developing non-invasive methods and other techniques, presence of advanced healthcare infrastructure, surge in regulatory approvals, focus of manufacturers on diversifying product portfolios and expanding services as well as significant healthcare expenditure are the factors driving the growth of the U.S. intra-abdominal pressure measurement devices market.
| Report Coverage | Details |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product, Procedure, Application, End user, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Increased cases related to abdominal problems
The growing number of patient admissions in intensive care units (ICUs) as well as critically ill patients are at high risk of developing IAH which can lead to severe ACS, creating a need for accurate and reliable IAP monitoring devices enabling early diagnosis for providing timely treatments to patients. Furthermore, increased incidences of trauma injuries and abdominal distress as well as huge number of surgical procedures are driving the adoption of IAP measurement devices in various healthcare settings.
Limited awareness among healthcare professionals
Despite the increased awareness regarding the severe effects and complications associated with IAH and ACS for critically ill patients’ in healthcare professionals, a lack of understanding and standardized guidelines for IAP monitoring in several healthcare settings as well as insufficient training and perceived complexity on the implementation of IAP measurement devices among healthcare workers is leading to underuse of these devices for patients.
Ongoing technological advancements
Medical device manufacturers are highly focused on developing innovative techniques, methods and devices for IAP monitoring. Huge investments in R&D activities, emphasis on expanding product portfolios and expanding market reach, development of non-invasive methods such as muscle contraction (MC) sensors and increased clinical applications of these devices are creating opportunities for market growth. Additionally, increased approvals from the Food and Drug Administration (FDA) supporting product launches and accelerating time to market reach are leading to increased involvement of major manufacturers as well as new market players fostering innovation.
The equipment segment captured the largest market share in 2024. The ongoing technological advancements like device miniaturization, improvements in sensor technology and development of real-time monitoring devices integrated with AI and machine learning as well as development of non-invasive measurement techniques enhancing user interface are driving the adoption of equipment. Moreover, need for early diagnosis and management, increased healthcare expenditure, integration of IAP monitoring devices with other monitoring systems for enhancing clinical decisions, and growing awareness regarding the complications associated with intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) among medical professionals is driving the market growth.
The disposables segment is anticipated to grow at the fastest rate during the forecast period. Disposables offer a cost-effective solution for IAP measurement, further eliminating the need for sterilization and reprocessing, particularly in large-scale settings. Use of single-use disposable products enhances patient safety by reducing the risk of cross-contamination and nosocomial infections in healthcare facilities. Furthermore, increased number of trauma and abdominal surgeries, enhanced biocompatibility and durability of disposable products such as pressure monitoring catheters, and development of di-(2-ethylhexyl) phthalate (DEHP)-free disposable devices are the factors contributing to the market growth of this segment.
The abdomen segment dominated the market with the largest share in 2024. The growing prevalence of abdominal surgeries using open and minimally invasive techniques, increased awareness regarding the complications associated with IAH and ACS among healthcare professionals, rising incidences of acute abdominal syndromes such as acute pancreatitis and intestinal perforation, and need for accurate and efficient IAP monitoring systems are the factors driving the market growth of this segment.
The muscle segment is anticipated to witness lucrative growth over the forecast period. The market growth of this segment is driven by the increased sport activities and physical fitness trends among individuals leading to rise in number of muscle-related injuries which requires proper monitoring of intra- compartment pressure in serious cases to prevent further complications such as compartment syndrome. Abdominal muscle surgeries include laparoscopic surgeries, abdominoplasty, mesh plasty, temporary abdominal closure (TAC) techniques as well as decompressive laparotomy for ACS which use intra-abdominal pressure devices like pressure transducers and indirect methods like monitoring intravesical pressure.
The intra-abdominal hypertension (IAH) segment accounted for the largest market share in 2024. Increased research activities for the development of precise and continuous IAP monitoring techniques such as automated monitoring systems and point-of-care ultrasound (POCT) for IAP evaluation, focus on exploring potential biomarkers for early identification of IAH and ACS, use of personalized treatment strategies such as neuromuscular blockade and fluid management, need for development of pediatric-specific diagnostic and management guidelines for IAH as well as growing emphasis on understanding IAH pathophysiology are the factors fostering the market expansion. Furthermore, the influence of the World Society for the Abdominal Compartment Syndrome (WSACS) promotes the research and development of guidelines for management of IAH and ACS management.
The intra-compartment pressure segment is expected to grow at the fastest rate during the predicted timeframe. The market growth of this segment is driven by the rising cases of orthopaedic and trauma injuries, growing awareness regarding serious consequences related with intra-compartment pressure such as disability and muscle damage as well as increased adoption in rehabilitation and sports medicine due to growing number of muscle-related injuries leading to compartment syndrome. Ongoing advancements in intra-compartmental pressure measurement such as non-invasive techniques like shear wave elastography and innovative digital continuous sensors are enhancing the reliability and proficiency of diagnosis and management of compartment syndrome.
The hospitals segment held the largest market share in 2024. The market dominance of this segment can be attributed to the increased rates of intra-abdominal hypertension (IAH) and abdominal compartment syndrome cases, surging number of ICU admissions, growing focus on integrating standardised protocols for IAP monitoring enabling early diagnosis and timely interventions for IAH, adoption of advanced continuous IAP monitoring systems such as capsular sensors and Serenno System as well as emphasis on improving patient safety and treatment outcomes.
The trauma centers segment is anticipated to grow at a notable rate over the forecast period. In 2024, according to the National Trauma Data Bank, about 37 million individuals experienced a trauma-related event leading to emergency department visit and approximately 2.6 million individuals were hospitalized due to traumatic injuries in the U.S. Trauma centers are focused on enhancing patient safety by offering services such as early diagnosis of IAH and ACS for reducing morbidity and mortality, building partnerships with hospitals for developing collaborative research initiatives and demonstrating the cost-effectiveness of IAP monitoring for patients ultimately leading to a supportive healthcare infrastructure.

By Product
By Procedure
By Application
By End User
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
February 2024
September 2024
April 2025
January 2025