January 2025
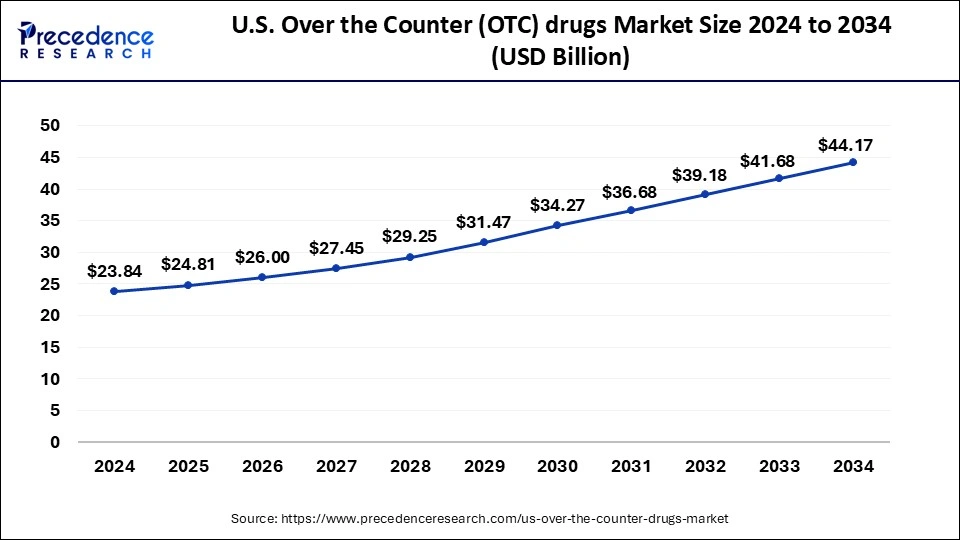
The U.S. over the counter (OTC) drugs market size accounted for USD 24.81 billion in 2025 and is forecasted to hit around USD 44.17 billion by 2034, representing a CAGR of 6.60% from 2025 to 2034. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The U.S. over the counter (OTC) drugs market size was estimated at USD 23.84 billion in 2024 and is predicted to increase from USD 24.81 billion in 2025 to approximately USD 44.17 billion by 2034, expanding at a CAGR of 6.60% from 2025 to 2034.
Over the counter drugs refer to medications that are available for purchase without a prescription from a healthcare professional. These drugs are also known as non-prescription drugs and are typically found on the shelves of pharmacies, supermarkets, and convenience stores. Unlike prescription drugs, which require a healthcare provider's authorization, OTC drugs can be bought directly by consumers for self-medication.
OTC drugs are generally considered safe when used as directed and for the intended purpose. They are commonly used to treat minor health conditions such as headaches, cold and flu symptoms, allergies, and mild pain. Common examples of OTC drugs include pain relievers like acetaminophen and ibuprofen, cough and cold medications, antacids, allergy medications, and topical creams for skin conditions.
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 6.60% |
| Market Size in 2025 | USD 24.81 Billion |
| Market Size by 2034 | USD 44.17 Billion |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product Type, Dosage Form, Route of Administration, and Distribution Channel |
Increasing prevalence of various diseases
Acute and chronic illnesses such as respiratory, neurological, orthopedic, and cardiovascular conditions are becoming more prevalent in the US. Every year in the US, heart failure is diagnosed in over 550,000 persons. The Centers for Disease Control and Prevention (CDC) estimate that every year, over 790,000 Americans suffer from a heart attack; of them, about 580,000 experience their first heart attack and 210,000 experience their second. Diabetes, hypertension, tobacco use, obesity, and other risk factors are making chronic cardiac, neurological, and orthopedic illnesses more common.
As the prevalence of chronic illnesses has increased, so has the usage of medications as people look for easy and efficient ways to manage their conditions. For instance, aspirin is frequently used in people with a history of heart disease or who are at high risk of developing heart disease to prevent heart attacks, strokes, and other cardiovascular disorders. In the same way, acetaminophen and ibuprofen are frequently used to lessen pain and inflammation brought on by ailments including headaches, chronic pain in the back, and arthritis. Thus, the increasing prevalence of various diseases drives the U.S. over the counter (OTC) drugs market.
Safety concerns and regulatory challenge
OTC medications are typically regarded as safe when used as prescribed, however, abuse or overuse of them may raise safety issues. Making sure that products are safe for consumers is a continuous problem since certain components may have negative effects or interact with other treatments.
Furthermore, the market for over the counter drugs is governed by rules, and modifications to these regulations may affect the accessibility and promotion of certain OTC drugs. Regulatory obstacles may impact the status of already-approved OTC medications or delay the launch of new ones. Therefore, safety concerns and regulatory challenges might be a major impeding factor to the U.S. over the counter (OTC) drugs market's growth.
Growing approvals
The increasing approvals in the industry are expected to offer an attractive opportunity for market growth during the forecast period. For instance, in August 2023, the U.S. Food and Drug Administration (FDA) authorized ZURZUVAETM (zuranolone) 50 mg for people with postpartum depression (PPD), according to a statement from Biogen Inc. and Sage Therapeutics, Inc. For women with PPD, ZURZUVAE is the first and only oral, once-daily, 14-day medication that can significantly reduce depression symptoms.
Furthermore, a Complete Response Letter (CRL) was released by the FDA about the New Drug Application (NDA) for zuranolone, which is intended to treat the major depressive disorder (MDD) in adults. The CRL declared that further research or studies would be required since the application did not offer sufficient proof of efficacy to justify the approval of zuranolone for the treatment of MDD. Sage and Biogen are analyzing the comments and planning the next phase of action.
The cough & cold products segment held the largest share of the U.S. over the counter (OTC) drugs market in 2024. A wide variety of viruses can cause the common cold. The illness is usually harmless, and symptoms go away in two weeks on average. The growth in over the counter (OTC) medicine use in this category can be attributed to the rise in the number of persons who are afflicted with cough, cold, and flu. As a last option, consumers rely on over-the-counter medications for cough, cold, or flu symptoms.
The tablets segment held the largest share of the U.S. over the counter (OTC) drugs market in 2024. Over the counter tablets are available in a wide range of therapeutic areas, such as digestive health, allergy relief, cold and flu treatment, pain management, and more. To treat various ailments, tablets may contain a single active ingredient or a mixture of active compounds. Additionally, customers frequently choose tablets for self-medication since they are a practical and simple dosing type. They may be taken without water in some situations due to their discreet and portable design, which makes them popular.
The oral segment held the dominating share of the market in 2024. The segment is observed to sustain the position in the upcoming period. Oral drugs provide a convenient method of medication delivery, allowing patients to take their medication at home or on the go without the need for medical supervision. This convenience promotes better adherence to treatment plans.
In addition, compared to some other routes of administration (such as injections or intravenous infusions), taking medications orally is generally less invasive and more comfortable for patients. Thus, these advantages drive the segment expansion.
The drug stores & retail pharmacies segment held the largest share of the U.S. over the counter (OTC) drugs market in 2024. Drug stores and retail pharmacies provide a diverse range of OTC drugs that cater to different health needs. This includes pain relievers, cough and cold medications, allergy remedies, digestive aids, vitamins, and more. Thus, the availability of these products allows consumers to address common health concerns without visiting a healthcare provider for a prescription.
By Product Type
By Dosage Form
By Route of Administration
By Distribution Channel
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
January 2025
March 2025
March 2025
October 2024