December 2024
The global bladder cancer therapeutics diagnostics market size is calculated at USD 5.68 billion in 2025 and is forecasted to reach around USD 9.53 billion by 2034, accelerating at a CAGR of 5.92% from 2025 to 2034. The North America market size surpassed USD 2.25 billion in 2024 and is expanding at a CAGR of 6.05% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
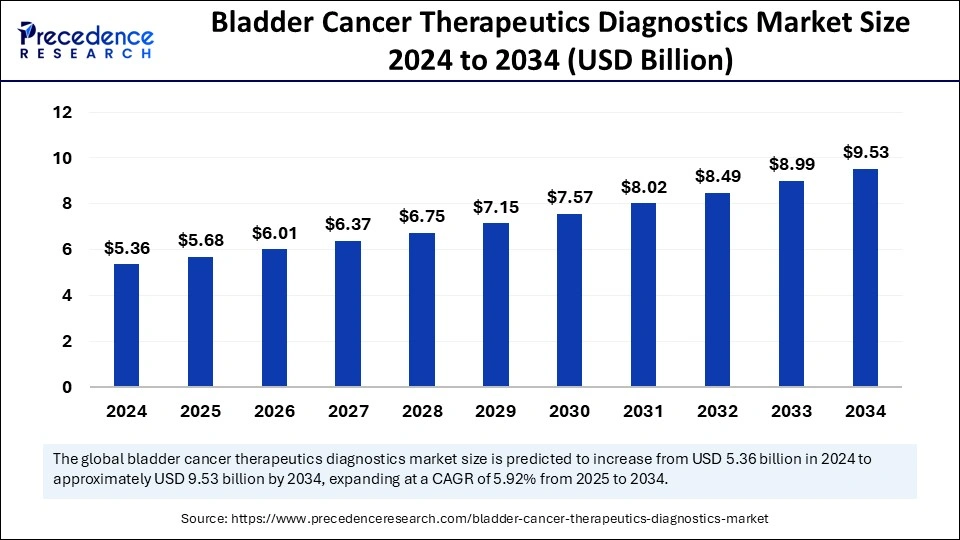
The global bladder cancer therapeutics diagnostics market size accounted for USD 5.36 billion in 2024 and is predicted to increase from USD 5.68 billion in 2025 to approximately USD 9.53 billion by 2034, expanding at a CAGR of 5.92% from 2025 to 2034. The market is witnessing considerable expansion owing to the increasing prevalence and awareness of bladder cancer. Progress in precision medicine and tailored treatment methods, coupled with non-invasive diagnostic tools, is fostering this growth.
Artificial intelligence (AI) is transforming the bladder cancer therapeutics diagnostics market by enhancing diagnostic precision, customizing treatment plans, and forecasting patient outcomes. AI algorithms support tumor detection and staging, provide real-time assistance during cystoscopies, offer predictive analytics for the likelihood of cancer recurrence and treatment efficacy, and enable digital pathology for grading and predicting molecular subtypes. These applications improve diagnostic precision, tailored treatment methods, and patient outcomes.
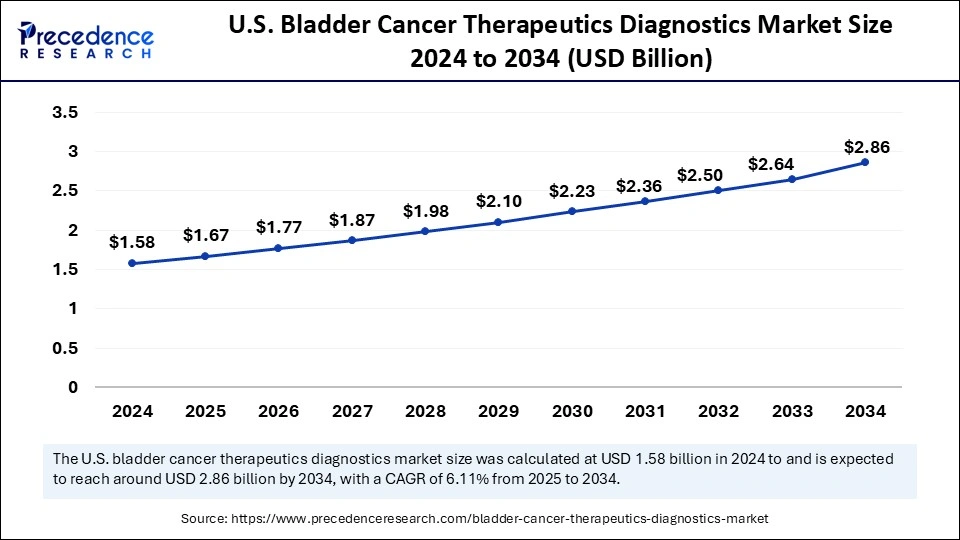
The U.S. bladder cancer therapeutics diagnostics market size was exhibited at USD 1.58 billion in 2024 and is projected to be worth around USD 2.86 billion by 2034, growing at a CAGR of 6.11% from 2025 to 2034.
Bladder Cancer Prevalence Drives Market Dominance in North America
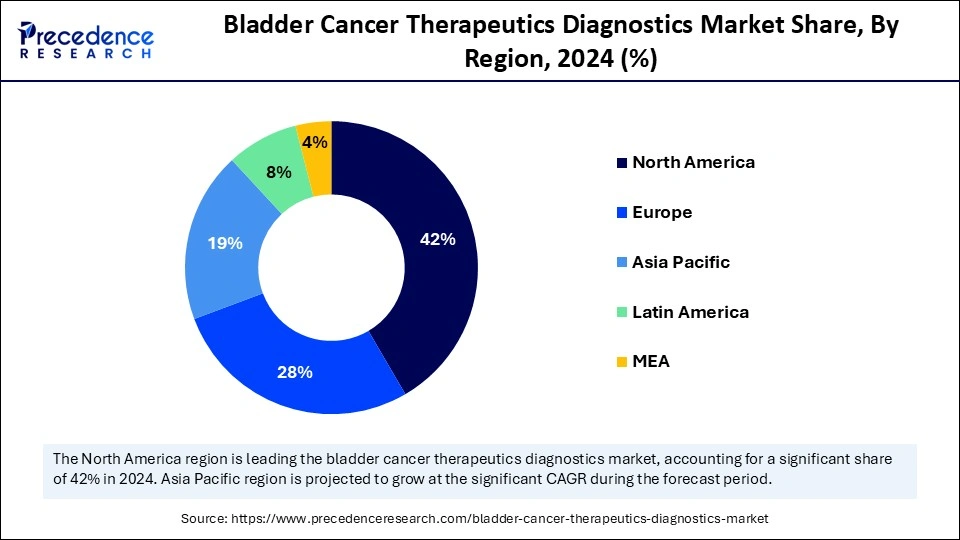
North America dominated the bladder cancer therapeutics diagnostics market in 2024 because of its high prevalence, sophisticated healthcare systems, and substantial research funding. The U.S., due to its expanding elderly population and heightened awareness of bladder cancer signs, occupies a substantial portion of the market. The elevated incidence rate demands efficient diagnostic methods and treatment choices. The existence of leading pharmaceutical firms and research organizations in North America creates a setting favorable for innovation, resulting in the swift emergence of new treatments. Access to financial resources, such as venture capital and private equity, enhances the development and commercialization of innovative solutions.
The U.S. Market Trends
The U.S. excels in the market due to its sophisticated healthcare system, ample research financing, and prominence in innovation and clinical studies. The National Institutes of Health and the National Cancer Institute offer significant financial support for bladder cancer research, as the market is strengthened by a dynamic innovation ecosystem. The considerable frequency of bladder cancer and heightened patient awareness boost the need for innovative treatments.
Asia Pacific: A Future Hub for Bladder Cancer Therapeutics and Diagnostics
Asia Pacific is anticipated to grow at the fastest rate in the bladder cancer therapeutics diagnostics market during the forecast period, driven by various factors such as a growing elderly population, rising healthcare spending, and notable progress in diagnostic technologies. The demographic trend towards an aging population is especially noticeable in nations such as Japan, where around 28.7% of residents are over 65 years old, leading to an increased need for bladder cancer screening and care. Moreover, progress in technology has resulted in an increase in authorized diagnostic tools. This innovation is fostering market growth by offering sophisticated solutions for the early identification and treatment of bladder cancer.
Advanced Diagnostics Propel Japan Forward
Japan is becoming a crucial participant in the market because of its elevated prevalence rates and sophisticated healthcare system. The focus of the nation on prompt detection and treatment, along with substantial funding in oncology, has resulted in the use of advanced diagnostic technologies such as fluorescence cystoscopy and urine-based biomarkers. The recent launch by Japan of innovative immunotherapies and targeted therapies has improved treatment results by establishing it as a frontrunner in bladder cancer care.
Bladder Cancer Care in China Is Booming
China is witnessing swift expansion in the market driven by increasing incidence and healthcare funding. The nation is experiencing an increasing challenge of bladder cancer because of urbanization, pollution, and changes in lifestyle. The government has focused on cancer control programs and enhanced access to advanced medical technologies. Innovations comprise urine biomarker tests that improve early identification and treatment effectiveness. Partnerships between domestic biotech companies and global pharmaceutical corporations strengthen the status of China in this market.
Innovative Diagnostics Drive the Market Position of Europe
The European market is growing at a considerable rate owing to its significant prevalence and progress in diagnostic technologies. Government programs encourage early identification and treatment, while the elderly demographic heightens awareness. Advanced diagnostic methods such as liquid biopsy and urine tests are being utilized to boost early identification and enhance patient results.
Bladder cancer is a prevalent cancer that impacts the urinary bladder, with transitional cell carcinoma being the most frequent form. The market for bladder cancer therapeutics and diagnostics is an expanding field concentrating on solutions and services for the treatment and diagnosis of bladder cancer. This market encompasses treatments such as chemotherapy and immunotherapy, along with diagnostics like cystoscopy, bladder ultrasound, and urinalysis.
The bladder cancer therapeutics diagnostics market is anticipated to witness considerable expansion owing to the advancement of targeted treatments, imaging technologies, urine-based indicators, and biopsy methods. The market comprises pharmaceutical firms, diagnostic producers, research organizations, and healthcare professionals. The rising prevalence of bladder cancer worldwide, along with technological progress in diagnostics and treatments, has established this market as an essential area in cancer care.
| Report Coverage | Details |
| Market Size by 2034 | USD 9.53 Billion |
| Market Size in 2025 | USD 5.68 Billion |
| Market Size in 2024 | USD 5.36 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 5.92% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Type of Diagnostics, Treatment Modalities, Stage of Cancer, End User, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Emergence of immunotherapies
The emergence of immunotherapies is a major driver in the bladder cancer therapeutics diagnostics market, as it transforms cancer care by focusing on and eliminating cancer cells. This method is especially beneficial for patients with advanced or recurring bladder cancer, providing improved results and the possibility of minimizing side effects in comparison to conventional therapies such as chemotherapy.
High cost of treatments and diagnostics
The high cost of bladder cancer treatments and diagnostics presents a major restraint in the bladder cancer therapeutics diagnostics market, establishing financial challenges for patients and healthcare providers. Progress in treatments has enhanced results, yet their substantial expense renders them unavailable to numerous patients, particularly in low and middle-income nations. The persistent nature of bladder cancer, characterized by elevated recurrence rates, requires ongoing management. Advanced therapies like immunotherapies and targeted interventions typically involve significant costs, which further intensify the overall expense.
Adoption of non-invasive diagnostic technologies
The adoption of liquid biopsies provides a non-invasive option to conventional diagnostic approaches, enabling the early identification of cancer-associated biomarkers in bodily fluids such as blood or urine. This method minimizes discomfort and risk, especially advantageous for patients with bladder cancer. Liquid biopsies allow for ongoing tracking of treatment responses and disease advancement, helping clinicians make educated choices regarding patient care.
The imaging techniques segment dominated the bladder cancer therapeutics diagnostics market with the highest share in 2024, facilitating precise identification and assessment of the condition. Cystoscopy is considered the gold standard for diagnosing bladder cancer because of its excellent sensitivity and specificity. Sophisticated imaging techniques such as magnetic resonance imaging and computerized tomography scans are being combined with AI technologies to improve diagnostic abilities. These methods enhance anatomical visualization while also aiding in tumor staging and treatment response evaluations, leading to better patient outcomes. Recent progress and partnerships enhance their leadership in the market.
The urinary biomarkers segment is projected to expand rapidly in the coming years due to their non-invasive approach, affordability, and ability to identify the disease early. These tests, such as FDA-approved options like NMP22, BTA stat, and UBC, provide a viable alternative to conventional invasive methods. Their ease of use, great sensitivity, and capability to simplify diagnostic procedures make them favored by healthcare professionals and patients.
The immunotherapy segment held a dominant presence in the bladder cancer therapeutics diagnostics market in 2024 because it utilizes the immune system of the body to efficiently identify and eliminate cancer cells. Immunotherapies, especially those aimed at immune checkpoints, have demonstrated considerable effectiveness in managing different stages of bladder cancer, particularly in individuals with metastatic urothelial carcinoma and non-muscle invasive bladder cancer. Recent developments, including immune checkpoint inhibitors like atezolizumab, nivolumab, and pembrolizumab, have broadened treatment choices for patients with BCG-refractory or advanced disease, enhancing survival rates and offering lasting responses.
The radiation therapy segment is expected to witness the fastest growth during the predicted timeframe due to technological advancements, organ-preserving treatment approaches, and its efficacy when combined with other methods. Advancements such as Intensity-Modulated Radiation Therapy (IMRT) and Stereotactic Ablative Radiotherapy (SABR) have enhanced treatment accuracy and effectiveness, allowing for increased radiation doses at tumor locations. Multi-modality methods, especially for muscle invasive bladder cancer, are becoming increasingly popular. Tailored radiotherapy and the incorporation of radioprotective proteins are essential elements driving this expansion.
The non-muscle invasive segment held a significant share in the bladder cancer therapeutics diagnostics market in 2024, owing to its widespread occurrence and the necessity for early detection and treatment approaches. Non-muscle invasive bladder cancer makes up 70-80% of all bladder cancer instances, positioning it as a key area for research and therapy. The market is anticipated to expand considerably, owing to funding in research, regulatory certifications, and new treatments focused on lowering recurrence rates and enhancing patient outcomes. The elevated prevalence of non-muscle invasive bladder cancer requires targeted diagnostic and therapeutic strategies.
The muscle-invasive bladder cancer segment is projected to grow with the fastest CAGR during the forecast period because of its rising incidence and progress in treatment options. Muscle-invasive bladder cancer represents a substantial share of advanced cases, requiring more aggressive treatments such as radical cystectomy, chemotherapy, and immunotherapy. The growing prevalence of muscle-invasive bladder cancer, particularly in older individuals and those with risk factors such as smoking, has resulted in heightened awareness and a greater need for effective therapies.
The hospitals segment dominated the bladder cancer therapeutics diagnostics market with the largest share in 2024 because of their extensive care capabilities, access to advanced technologies, multidisciplinary skills, and ability to conduct specialized treatments. This includes advanced tools like cystoscopy and imaging technologies, along with their capacity for surgery, chemotherapy, and radiation therapy. This supremacy is propelled by the rising incidence of bladder cancer and the demand for sophisticated diagnostic techniques for early identification and monitoring.
The ambulatory surgical centers segment will grow at the fastest rate in the upcoming years, driven by rising demand for outpatient services, minimally invasive surgical methods, and economical healthcare options. This segment provides convenient and effective environments for bladder cancer treatments like cystoscopies and biopsies, lowering healthcare expenses and enhancing patient satisfaction. Technological advancements allow ambulatory surgical centers to carry out intricate procedures with great accuracy and safety by making them appealing choices for patients and healthcare professionals alike.

By Type of Diagnostics
By Treatment Modalities
By Stage of Cancer
By End User
By Region
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
December 2024
January 2025
September 2024
September 2024