March 2025
The global companion diagnostics market size accounted for USD 8.87 billion in 2024, grew to USD 8.87 billion in 2025 and is projected to surpass around USD 28.54 billion by 2034, representing a healthy CAGR of 12.40% between 2024 and 2034. The North America companion diagnostics market size is calculated at USD 2.86 billion in 2024 and is expected to grow at a fastest CAGR of 12.51% during the forecast year.
The global companion diagnostics market size is estimated at USD 8.87 billion in 2024 and is anticipated to reach around USD 28.54 billion by 2034, expanding at a CAGR of 12.40% between 2024 and 2034.
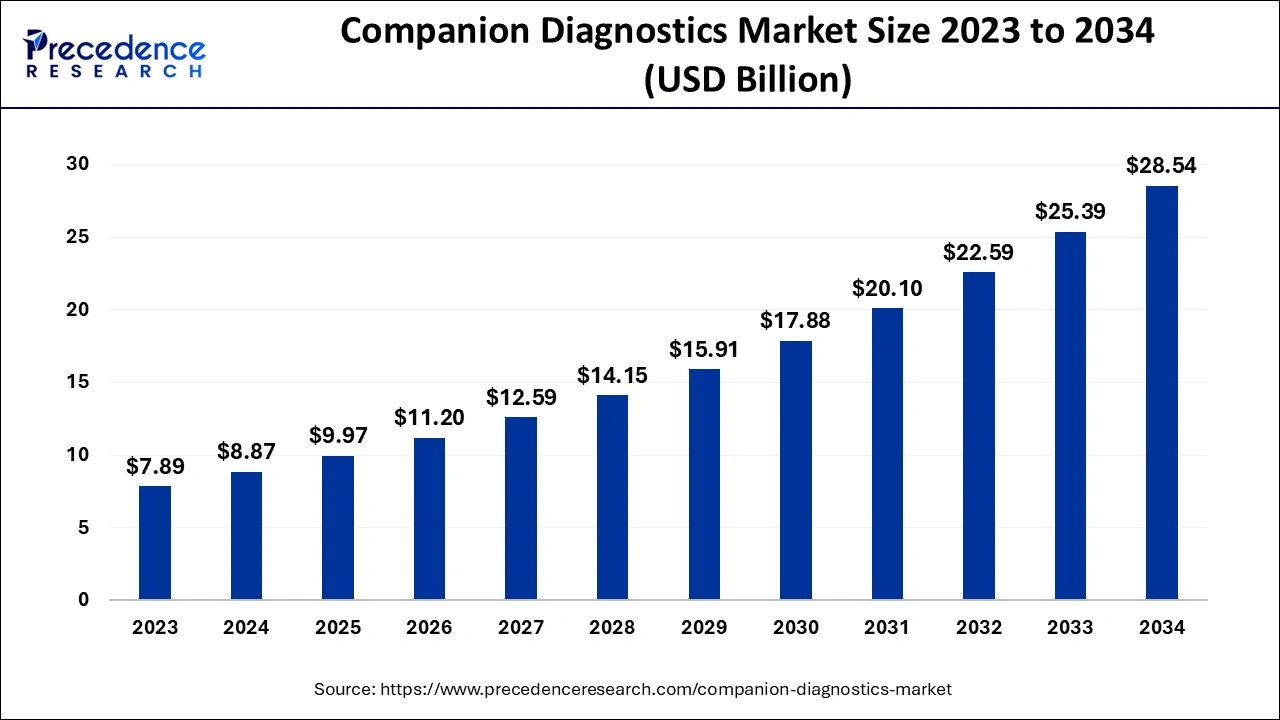
The U.S. companion diagnostics market size is estimated at USD 4.08 billion in 2024 and is anticipated to reach around USD 13.27 billion by 2034, exxpanding at a CAGR of 12.59% between 2024 and 2034.
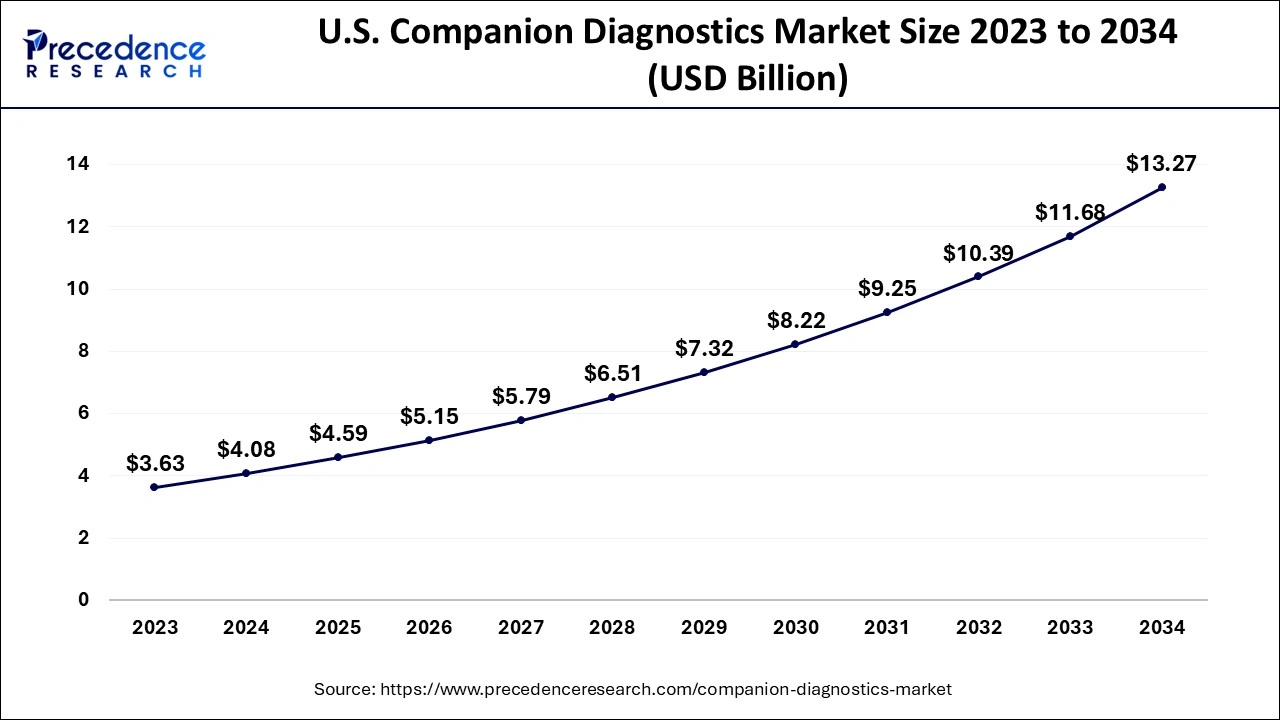
North America represented the biggest portion of the friend diagnostics market. The North American friend diagnostics market development can be ascribed to the presence of many driving companion diagnostics sellers and public clinical research centers, the simple availability of innovatively progressed gadgets and instruments, and the profoundly evolved medical services framework in the US and Canada.
The utilization of companion diagnostics is viewed as a significant treatment choice device for different oncology drugs, which is likewise reflected in how the FDA orders these examinations concerning risk.
The companion diagnostics clinical preliminaries have come to the cutting edge in the drug business, regardless of the COVID-19 flare-up, as it helps support the possibilities of clinical achievement. The testing unit has been seeing popularity for distinguishing those contaminated with SARS-CoV-2. The novel Covid stayed the primary concentration for symptomatic test producers regarding innovative work.
The rising weight of disease in the United States is additionally expected to drive market development. As indicated by the information distributed by American Cancer Society Inc., in January 2020, in the United States, there were around 1.8 million new disease cases analyzed and 606,520 malignant growth passings.
Moreover, the great repayment situation for bosom disease symptomatic arrangements in the United States is expected to work with their reception. For example, in January 2020, the Centers for Medicare and Medicaid Services (CMS) extended its inclusion of cutting-edge sequencing as an asymptomatic device for patients with germline (acquired) bosom malignant growth.
Additionally, in October 2021, the United States Food and Drug Administration endorsed Agilent's Ki-67 IHC MIB-1 pharmDx (Dako Omnis) that guides in recognizing patients with early bosom malignant growth (EBC) at high gamble of infection repeat. This will additionally prompt drive the market development over the conjecture period around here.
Inferable from the above factors, the North American portion is supposed to hold a significant offer in the worldwide companion diagnostics market.
Asia-Pacific presents rewarding open doors for the central members working in the companion diagnostics market, attributable to high populace base, development in mindfulness about companion diagnostics, improvement in medical care foundation, and expansion popular for advanced treatments. organizations are zeroing in on consolidations and acquisitions to reinforce the improvement in indicative methods, as would be considered normal to drive the market development around here. Nonetheless, greater expenses caused in R&D to create friend diagnostics can hamper the companion diagnostics market development in Asia-Pacific.
Companion Diagnostics Market Growth Factors
With signs of progress in hereditary sequencing and genomics, it is currently generally accepted that medications can show shifting results in various people. A superior comprehension of the hereditary attributes or biomarkers of an individual can advance the act of overseeing 'the right medication, with impeccable timing, at the right portion, for the perfect individual'. Drug and biopharmaceutical organizations are ceaselessly endeavoring to execute patient-choice analytic systems in the previous phases of medication improvement to give designated treatments to the right competitor. These further backings the development of the companion diagnostics market.
NGS-based friend symptomatic tests intend to open atomic data from every patient's growth genome to direct therapy choices for disease treatments. Cutting-edge sequencing recognizes numerous biomarkers for various medication treatments in a more limited time when contrasted with other sequencing procedures. The utilization of NGS boards for biomarker estimation in one test can help in the therapy of a wide range of kinds of diseases. The different innovative headways in NGS likewise give market players a quick upper hand over players giving different advancements like PCR, ICH, and ISH. Subsequently, significant market players are zeroing in on creating companion demonstrative items in light of NGS.
| Report Coverage | Details |
| Market Size in 2024 | USD 8.87 Billion |
| Market Size by 2034 | USD 28.54 Billion |
| Growth Rate from 2024 to 2034 | CAGR of 28.54% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2034 |
| Segments Covered |
|
| Regions Covered |
|
Advancements in the latest technologies
The next-generation sequencing based companion indicative tests mean to open nuclear information from each persistent development genome to coordinate treatment decisions for illness medicines. Forefront sequencing perceives various biomarkers for different medicines in a more restricted time when stood out from other sequencing strategies. The use of NGS sheets for biomarker assessment in one test might perhaps help in the treatment of a wide scope of sorts of illnesses. The different creative degrees of progress in NGS moreover give market players a speedy advantage over players giving various headways like PCR, ICH, and ISH. Thusly, critical market players are focusing on making amigo illustrative things considering NGS.
Lack of awareness and high cost of companion diagnostics techniques
While immunotherapy has shown a critical guarantee in malignant growth treatment, its significant expense has restricted generally persistent access. Doctors could rather find it best to join treatments; this, thusly, could broaden the length of the treatment from five months to the north of three years. For this situation, the more extended the term, the more prominent the cost. Also, the expense of customized immunotherapies is high. The expense of disease immunotherapies can far surpass the expense expected for other treatment choices, like chemotherapy or radiation treatment. In this way, the significant expense of immuno-oncology treatments is supposed to hamper the development of the companion diagnostics market.
Opportunities: Growing advancements in biologics
The companion diagnostics market is exceptionally solidified. The top players are F. Hoffmann-La Roche Ltd. (Switzerland), Agilent Technologies, Inc. (the US), Qiagen N.V. (Germany), Thermo Fisher Scientific, Inc. (the US), Abbott Laboratories, Inc. (the US) - in the companion diagnostics market represented a joined larger part portion of the overall industry in 2020. There is a serious level of rivalry among the market players. Just significant organizations can bear the cost of high-capital ventures as well as the significant expense of R&D and production. This will keep new contestants from entering this market.
Lack of resources in underdeveloped nations
It is essential to assemble mindfulness among patients, clinicians, and the general population concerning the significance of clinical preliminaries. To further develop mindfulness among clinicians, giving extraordinary admittance to legitimate, proof put together most recent data concerning these new malignant growth medications can be useful. To work on open mindfulness, expanding admittance to a typical and believed wellspring of truth is vital. A few distributing houses and industry players give month-to-month diaries and data handouts. Partners have likewise centered around mindfulness drives to guarantee patients and suppliers can get to and figure out the advantages of friend diagnostics.
The companion diagnostics market is portioned into malignant growth, cardiovascular illnesses (CVDs), neurological sicknesses, irresistible infections, and different signs (provocative and acquired infections, among others). In 2022, the malignant growth section represented the biggest portion of the companion diagnostics market. Factors like developing job of friend diagnostics in the customized medication therapy for malignant growth, and expanding the utility of biomarkers in the analysis of disease.
The drug and biotechnology organizations and reference research facilities fragment represented the biggest offer on the lookout, by the end client. The companion diagnostics market is fragmented into drug and biotechnology organizations, reference labs, CROs, and opposite-end clients (counting doctor and clinic labs, and scholastic clinical focuses). In 2022, drug and biotechnology organizations represented a bigger portion of the companion diagnostics market. The developing utilization of companion diagnostics is inferable from their rising conspicuousness in drug improvement and the rising significance of companion analytic biomarkers.
By Indication
By Product & Service
By Technology
By Indication
By End-User
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
March 2025
June 2024
March 2025
December 2024