August 2024
Fabry Disease Treatment Market (By Treatment: Enzyme Replacement Therapy (ERT), Chaperone Treatment, Substrate Reduction Therapy (SRT), Others) - Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2024-2033
The global fabry disease treatment market size was valued at USD 2.10 billion in 2023 and is anticipated to reach around USD 4.54 billion by 2033, growing at a CAGR of 8.02% from 2024 to 2033. The global Fabry disease treatment market is poised for growth due to the escalating prevalence of this condition.
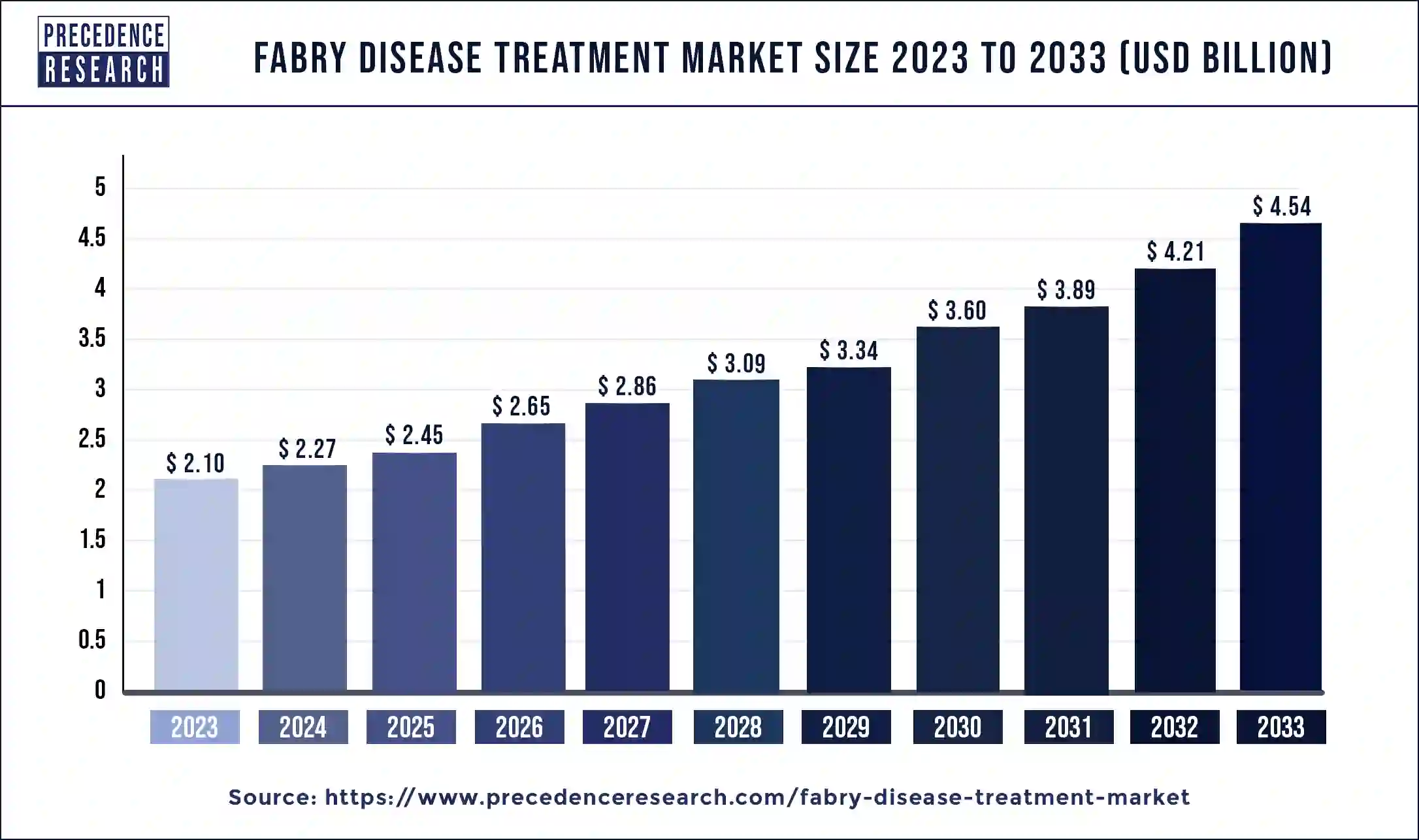
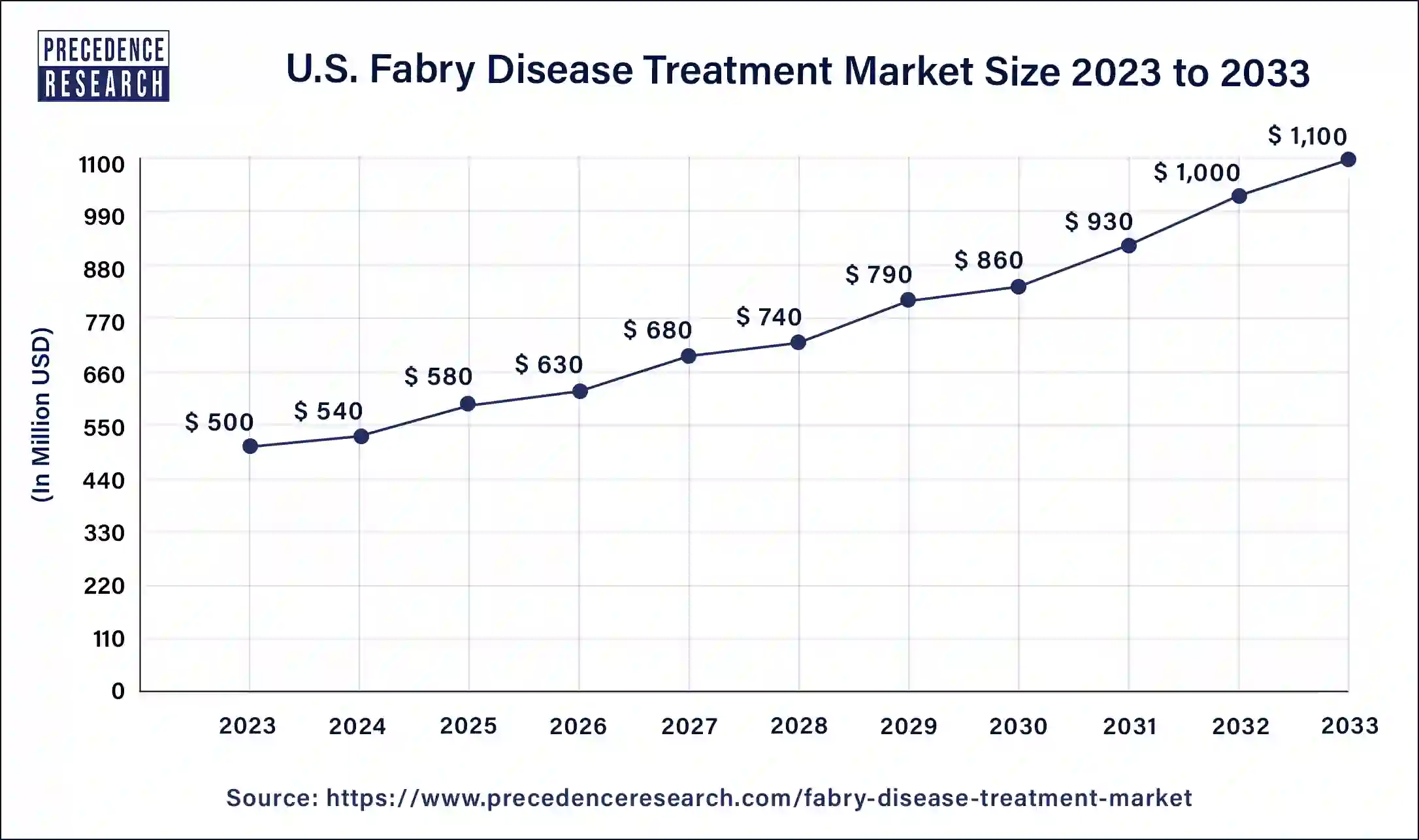
The U.S. fabry disease treatment market size was estimated at USD 500 million in 2023 and is predicted to be worth around USD 1,100 million by 2033 with a CAGR of 8.20% from 2024 to 2033.
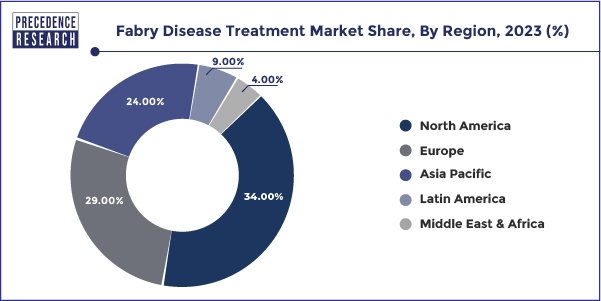
North America dominated the Fabry disease treatment market in 2023. The Fabry disease treatment market in the region is growing due to the higher adoption of novel therapies, advanced healthcare facilities, and favorable reimbursement policies. Health insurance programs covering expensive medications like Fabrazyme and supportive government healthcare policies are encouraging drug companies to invest more in R&D for rare diseases. Additionally, government initiatives to fund and support rare disease treatments are expected to drive market growth throughout the study period further.
Asia Pacific is expected to host the fastest-growing Fabry disease treatment market over the forecast period. Several factors are driving the market's growth during the forecast period, including a surge in the geriatric population, the development of a broad range of Fabry disease treatments, and improved accessibility to pharmaceutical products. Moreover, the rise in inherited neurological disorders, the presence of critical generic pharmaceutical companies, and increased government initiatives and specialized communities positively impact market growth. Significant advancements and improvements in Fabry disease treatment in the region further contribute to this growth.
The Asia Pacific fabry disease treatment market size was calculated at USD 500 million in 2023 and is projected to expand around USD 1,110 million by 2033, poised to grow at a CAGR of 8.30% from 2024 to 2033.
| Forecast Year | Market Size in USD |
| 2023 | $ 500 Million |
| 2024 | $ 540 Million |
| 2025 | $ 590 Million |
| 2026 | $ 640 Million |
| 2027 | $ 690 Million |
| 2028 | $ 740 Million |
| 2029 | $ 800 Million |
| 2030 | $ 860 Million |
| 2031 | $ 930 Million |
| 2032 | $ 1,010 Million |
| 2033 | $ 1,110 Million |
Fabry Disease Treatment Market Overview
Fabry disease is an inherited genetic disorder caused by the accumulation of globotriaosylceramide, a type of fat. Symptoms typically begin in childhood and include frequent pain, especially in the hands and feet, clusters of dark red spots on the skin known as angiokeratomas, cloudiness in the eyes, reduced sweating (hypohidrosis), and ringing in the ears. Patients may also experience hearing loss and gastrointestinal issues. Symptoms can vary widely among individuals, and some may exhibit other specific signs. Without treatment, Fabry's disease can lead to severe complications like stroke, heart failure, and progressive kidney failure. In some cases, symptoms are milder and appear later in adulthood, often affecting the blood vessels in the brain, kidneys, or heart.
| Report Coverage | Details |
| Fabry Disease Treatment Market Size in 2023 | USD 2.10 Billion |
| Fabry Disease Treatment Market Size in 2024 | USD 2.27 Billion |
| Fabry Disease Treatment Market Size by 2033 | USD 4.54 Billion |
| Fabry Disease Treatment Market Growth Rate | CAGR of 8.02% from 2024 to 2033 |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Driver: The increasing prevalence of Fabry disease
The global fabry disease treatment market is expected to grow due to the increasing prevalence of the condition. Research indicates that Fabry disease affects 1 in 1,000 to 9,000 people. As the population grows, the likelihood of this hereditary disorder being passed on to newborns also rises. It has been observed that milder symptoms are more familiar with age than the severe form.
Fabry disease can affect individuals of all ethnicities and genders, leading to a broader patient base. Moreover, growing awareness of Fabry disease and increased discussions in the scientific and medical communities have led to better understanding and earlier diagnosis of the condition, further driving the Fabry disease treatment market growth.
Restraint: Commodity price volatility and supply chain disruptions
Changes in the prices of raw materials, such as certain commodities, can affect the profit margins and operational costs of companies in the Fabry disease treatment market, exposing them to financial risks. Market saturation could also occur as similar products or services increase competition and put pressure on pricing and profitability, especially for companies with undifferentiated offerings.
Additionally, supply chain disruptions caused by natural disasters, geopolitical tensions, or other factors can hinder production and distribution, leading to delays, increased costs, and potential loss of market share in the Fabry disease treatment market.
Opportunity: Robust pipeline assets development
The Fabry disease treatment market is anticipated to experience significant growth in the coming years due to advancements in treatment options and a robust pipeline of assets developed by key market players. Factors contributing to this growth include increased awareness of Fabry disease treatment, a rise in research and development activities, and promising pipeline products.
Awareness of Fabry disease is crucial for both patients and healthcare professionals. Recently, awareness has been increasing in countries such as the United States and the United Kingdom. Furthermore, the surge in research and development efforts and the rise in funding for Fabry disease treatment are expected to drive the Fabry disease treatment market growth.
The Fabry disease treatment market in 2023 was dominated by the enzyme replacement therapy segment. Patients with Fabry disease can experience significant clinical benefits from this treatment, especially when it is started early, before organ damage sets in, such as chronic kidney disease or cardiac fibrosis. The demand for enzyme replacement therapies is expected to grow substantially due to their effectiveness in addressing the root cause of Fabry disease and their positive outcomes in patients. The two primary enzyme replacement therapies used in treating Fabry disease are Agalsidase Beta and Agalsidase Alfa. These therapies mimic the actions of alpha-galactosidase upon administration. Therapies like Fabrazyme directly target the underlying cause of Fabry disease and contribute to their increasing demand and anticipated market growth.
The substrate reduction therapy segment grows at a rapid pace in the Fabry disease treatment market over the forecast period. Substrate reduction therapy is named for its ability to reduce the levels of an enzyme's substrate. In Fabry disease, this therapy works by inhibiting the activity of the GCS enzyme by blocking the formation of GL-1 and preventing the production of Gb3, the substrate of alpha-galactosidase. By doing so, substrate reduction therapy effectively addresses the enzyme deficiency characteristic of Fabry disease, mitigating its impact.
Segments Covered in the Report
By Treatment
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
August 2024
January 2025
August 2024
January 2025