September 2024
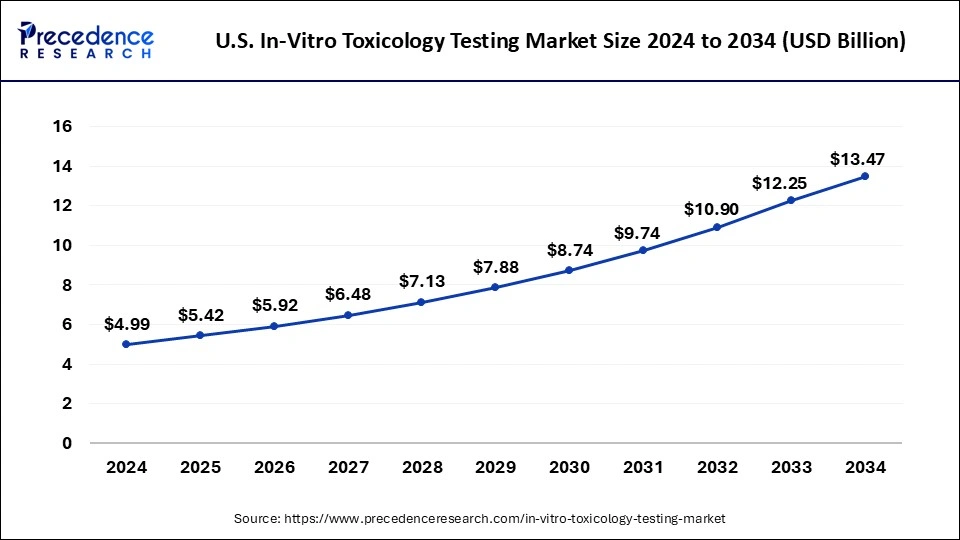
The global in-vitro toxicology testing market size accounted for USD 12.99 billion in 2025 and is forecasted to reach around USD 33.13 billion by 2034, accelerating at a CAGR of 10.97% from 2025 to 2034. The North America market size surpassed USD 5.61 billion in 2024 and is expanding at a CAGR of 10.76% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global in-vitro toxicology testing market size was calculated at USD 11.92 billion in 2024 and is predicted to increase from USD 12.99 billion in 2025 to approximately USD 33.13 billion by 2034, expanding at a CAGR of 10.97% from 2025 to 2034.
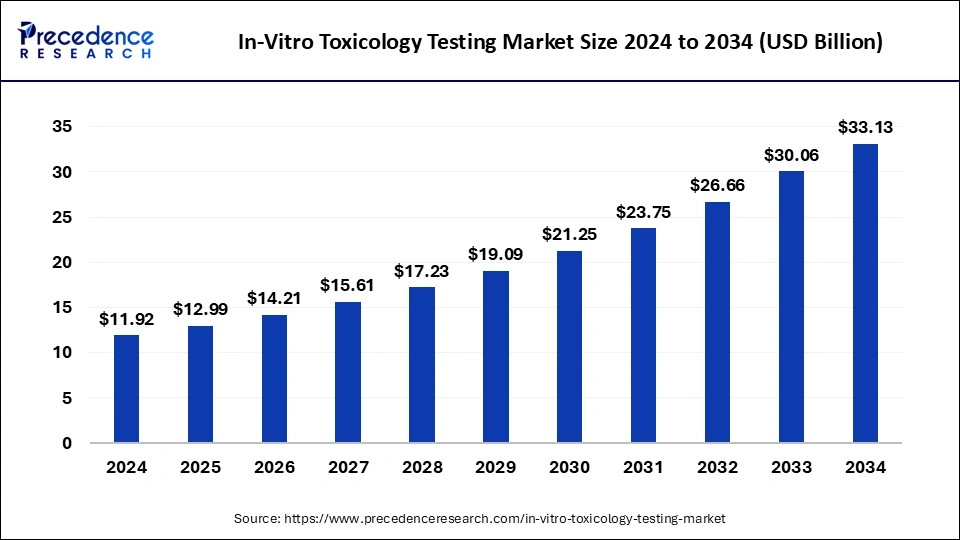
The U.S. in-vitro toxicology testing market size was exhibited at USD 4.99 billion in 2024 and is projected to be worth around USD 13.47 billion by 2034, growing at a CAGR of 10.64% from 2025 to 2034.
North America holds the largest share of the market. The region is expected to sustain its dominance during the forecast period owing to the sophisticated healthcare infrastructure, supportive government regulations, the increasing presence of prominent market players, advanced infrastructure & shifting focus on drug discovery in the region. Technological advancements and increasing adoption of in-vitro testing methods in the region also act as drivers for the growth of in-vitro toxicology testing market. The launch of biologics by biopharmaceutical players in the United States has resulted in the rapid adoption of toxicology testing. The expansion of laboratory capabilities in the region has enabled clients to build toxicological profiles of biopharmaceuticals, medical devices, chemicals, and cosmetics.
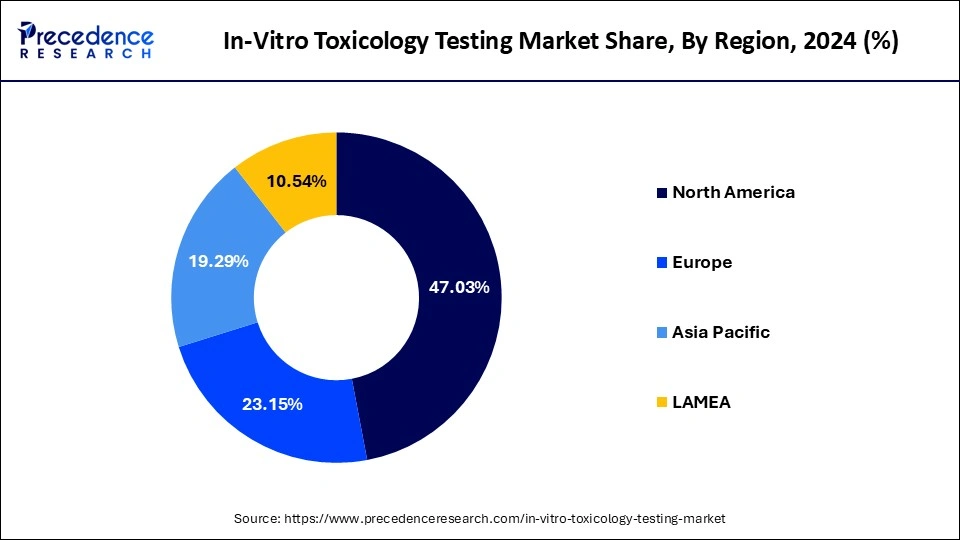
On the other hand, the Asia Pacific market is growing at a significant CAGR during the forecast period. The rapid growth of the market in the region is owing to the rapidly increasing geriatric population in need of medicines, several government incentives for enhancing technology and development, rising healthcare expenditure, and rising focus of government organisations to encourage toxicology testing by in-vitro methods. The conducting of clinical trials in the region is relatively cost-effective.
An in vitro test is conducted outside of a living organism. This study involves the use of isolated cells, tissues, or organs. In vitro generally means “in glass”, and it refers to methods that are conducted on living material or components of living material cultured in test tubes or Petri dishes under particular conditions. In-vitro assays offer toxicity information in a less expensive as well as time-saving manner.
In vitro methods for the assessment of toxicity are one of the alternatives to whole-animal testing procedures. In vitro toxicology tests are rapidly gaining popularity in the regulatory community as they can lessen the number of animals used while providing predictions for some toxicological endpoints. In vitro, toxicology screening methods are the major tools to reduce the attrition of novel drug candidates as they progress through the development and discovery process.
In vitro toxicity, assays are employed to identify the potential of a new agrochemical, pharmaceutical, food additive, or any other chemical product to be dangerous to humans. In vitro studies are performed on mammalian cells or cultured bacteria and can be used as a screening to avoid the unnecessary use of animals in determining which candidates should go ahead for further safety testing.
In-vitro toxicology tests are widely used to replace multiple studies that earlier have been performed or tested on animals. The development of physiology-relevant in vitro models has recently advanced, and this is promising for enhancing the translation of test results to predict negative consequences in humans. Chemical toxicity testing is shifting toward a human cell and organoid-based in vitro method for several reasons such as ethical rightfulness, scientific relevancy, cost-effectiveness, and efficiency. In vitro toxicity tests adopt the latest advancement in vitro toxicology to assist clients in identifying compound viability in the preclinical phase of new product discovery or drug development. In vitro, toxicology studies can assist in reducing liabilities linked with the failure of late stage in the drug discovery process.
The global in-vitro toxicology testing market is expected to grow significantly due to the rising demand for drug discovery. To identify the toxic ability of compounds early in the drug discovery process, several specialist scientific expertise is needed with tailored solutions in order to address specific toxicological liabilities. The high cost of animal testing to measure toxicity, coupled with the social & ethical concerns for these conventional tests, is expected to drive the growth of the global in-vitro toxicology testing market.
The growth of the global in-vitro toxicology testing market is driven by the developments in toxicology research, such as the use of 3D in-vitro models, increasing government initiatives, increasing awareness regarding drug product safety, alternatives to the use of animals in pre-clinical research, rising advancements in-vitro toxicology assays, increasing demand for in-vitro assays and toxicology testing along with the developments in these assays to measure the safety of diagnostics, drugs, cosmetics, and others. In addition, the primary reason for accelerating the growth of the market is to minimize the last-stage drug failure risk by in-vitro toxicology assays.
| Report Coverage | Details |
| Market Size in 2025 | USD 12.99 Billion |
| Market Size in 2024 | USD 11.92 Billion |
| Market Size by 2034 | USD 33.13 Billion |
| Growth Rate from 2025 to 2034 | CAGR of 10.97% |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Technology, Application, Method, and End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Increasing Governments and organizational support to avoid animal testing
The rising favorable government initiatives, which are highly concerned with banning animal testing and can lead to an increase in the adoption of in-vitro toxicology testing during the forecast period. Increasing investment by public and private agencies for the development of in-vitro test techniques. Funding programs are generally aimed to safeguard animal health, human health, and the environment by minimizing the dependency on animal models for the safe measurement of new chemical formulations and compounds.
Moreover, the increasing demand for cost-effective and safer alternatives to animal testing in the cosmetics, food, and pharmaceutical sectors acts as a primary fueling market revenue growth. The adoption of in-vitro toxicology testing is rapidly gaining immense popularity as these days people are becoming more aware and concerned about the adverse consequences of chemicals on both the environment and human health.
Lack of skilled professionals
The lack of skilled professionals is projected to hamper the global in-vitro toxicology testing market's growth. There is a requirement for skilled professionals to perform various activities such as experiments, analyzing data, and making observations. In addition, the less capability of in-vitro models to determine autoimmunity and immunostimulant is likely to limit the expansion of the global in-vitro toxicology testing market during the forecast period.
Technological advancement
The continuous advancements in technology, especially in the healthcare sector, are observed to offer a lucrative opportunity for the market’s growth during the forecast period. The rising penetration/deployment of technologies such as 3D cell culture models and high-throughput screening are observed to promote the market’s growth, which encourage the adoption of in-vitro toxicity testing services. Advanced technologies promise to help experts to develop more relevant and precise data on the toxicity of chemicals and reduce the dependency on animal testing.
Additionally, the automation of multiple systems is another advancement in the market to offer opportunities during the forecast period. The automation of systems allows proper data analysis. Technological advancements allow cost-effective and time-saving procedures for the manufacturers by boosting the speed of drug testing.
Based on the technology, the global in-vitro toxicology testing market is segmented into cell culture technology, high throughput technology, cellular imaging, and OMICS technology. The cell culture technology segment is expected to dominate the market over the forecast period. Advancements in human cell culture exposure enabled the development of in-vitro assay systems, which are demonstrative, highly predictive, and well-suited for toxicity screening of a wide range of chemicals. In-vitro toxicology includes using tissues or cells grown and maintained in an artificially controlled environment, outside of the natural environment to test the toxic attributes of several mixtures and compounds.
Global In-Vitro Toxicology Testing Market Revenue, By Technology, 2022-2024 (USD Million)
| Technology | 2022 | 2023 | 2024 |
| Cell Culture Tech | 4,416.5 | 4,756.6 | 5,415.3 |
| High Throughput Tech | 2,637.4 | 2,863.5 | 3,122.4 |
| Cellular Imaging | 2,077.7 | 2,249.3 | 2,445.7 |
| OMICS Tech | 1,044.7 | 1,120.3 | 1,206.6 |
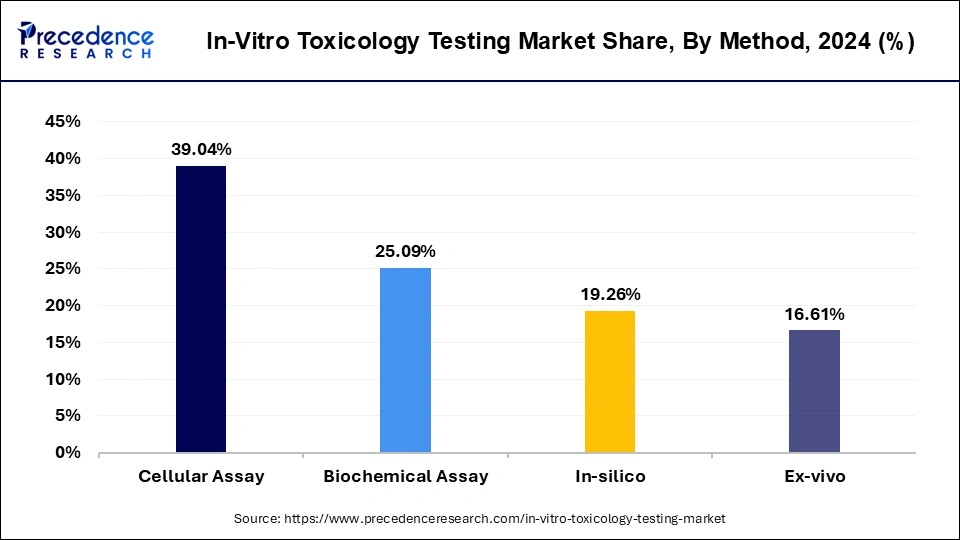
Based on the method, the global in-vitro toxicology testing market is segmented into cellular assay, biochemical assay, in-silico, and ex-vivo. The cellular assay segment is expected to hold a key account share during the forecast period. Cellular assays can be used to efficiently assess the cytotoxicity, biochemical mechanisms, off-target interactions, and biological activity in biomedical research as well as drug-discovery screening applications. Cellular assays are attributed to the high revenue in the in-vitro toxicology testing market. Cellular assays as in-vitro models provide various advantages including minimum cost, speed of analysis, and technological advancement such as automation. Moreover, several efforts by key market players for the development of novel cellular assays are expected to boost market growth. On the other hand, the In-silico segment is projected to grow at a significant CAGR during the forecast period.
Based on the application, the genotoxicity segment led the in-vitro toxicology testing market in 2023. The genotoxicity segment has emerged as the leading application in the in-vitro toxicology testing market. This dominance can be attributed to the increasing focus on assessing the potential genetic damage caused by various chemicals, pharmaceuticals, and environmental agents. Regulatory authorities, such as the FDA and EMA, have placed stringent requirements on genotoxicity testing to ensure the safety of drugs and other compounds, further driving demand in this segment.
Global In-Vitro Toxicology Testing Market Revenue, By Application, 2022-2024 (USD Million)
| Application | 2022 | 2023 | 2024 |
| Genotoxicity | 2,145.5 | 2,307.2 | 2,491.8 |
| Cytotoxicity | 1,835.7 | 1,980.7 | 2,146.6 |
| Phototoxicity | 574.7 | 620.5 | 673.0 |
| Carcinogenicity | 1,570.3 | 1,703.0 | 1,855.0 |
| Neurotoxicity | 1,029.7 | 1,105.2 | 1,191.5 |
| Dermal Toxicity | 905.9 | 993.9 | 1,094.9 |
| Endocrine Disruption | 944.0 | 1,014.8 | 1,095.6 |
| Ocular Toxicity | 754.1 | 819.5 | 894.4 |
| Others | 416.4 | 444.9 | 477.4 |
By Technology
By Application
By Method
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
September 2024
January 2025
February 2025
January 2025