April 2025
Protega Pharmaceuticals, approved by FDA, ROXYBOND™ a new 10 mg immediate-release oxycodone tablet. This is a big step toward solving the problem of current opioid abuse in the country. It is the first and sole FDA-approved abuse-deterrent formulation from misuse via intranasal and intravenous routes. It is designed using Protega's proprietary SentryBond™ technology, which is a new innovative emergence in opioid formulations and made it much harder to manipulate for misuse even with physical alteration. It has been designed for severe pain management among patients when the other alternatives will not suffice.

Protega Pharmaceuticals got an FDA nod on the ROXYBOND 10 mg formulation with SentryBond technology that combines inactive excipients with the active ingredient, oxycodone, to prevent manipulation and abuse while achieving the same release profile. It is also expected to mitigate overdose risk due to incomplete extraction, making it difficult to get access to unintended routes. While ROXYBOND will not resist various abuse methods, the design provides another critical layer of protection against misuse. It includes a significant tool for healthcare providers to fight the ongoing opioid crisis outside the clinical setting. The formulation would ideally provide a more secure alternative to the patient with severe pain but as little opportunity for abuse as possible.
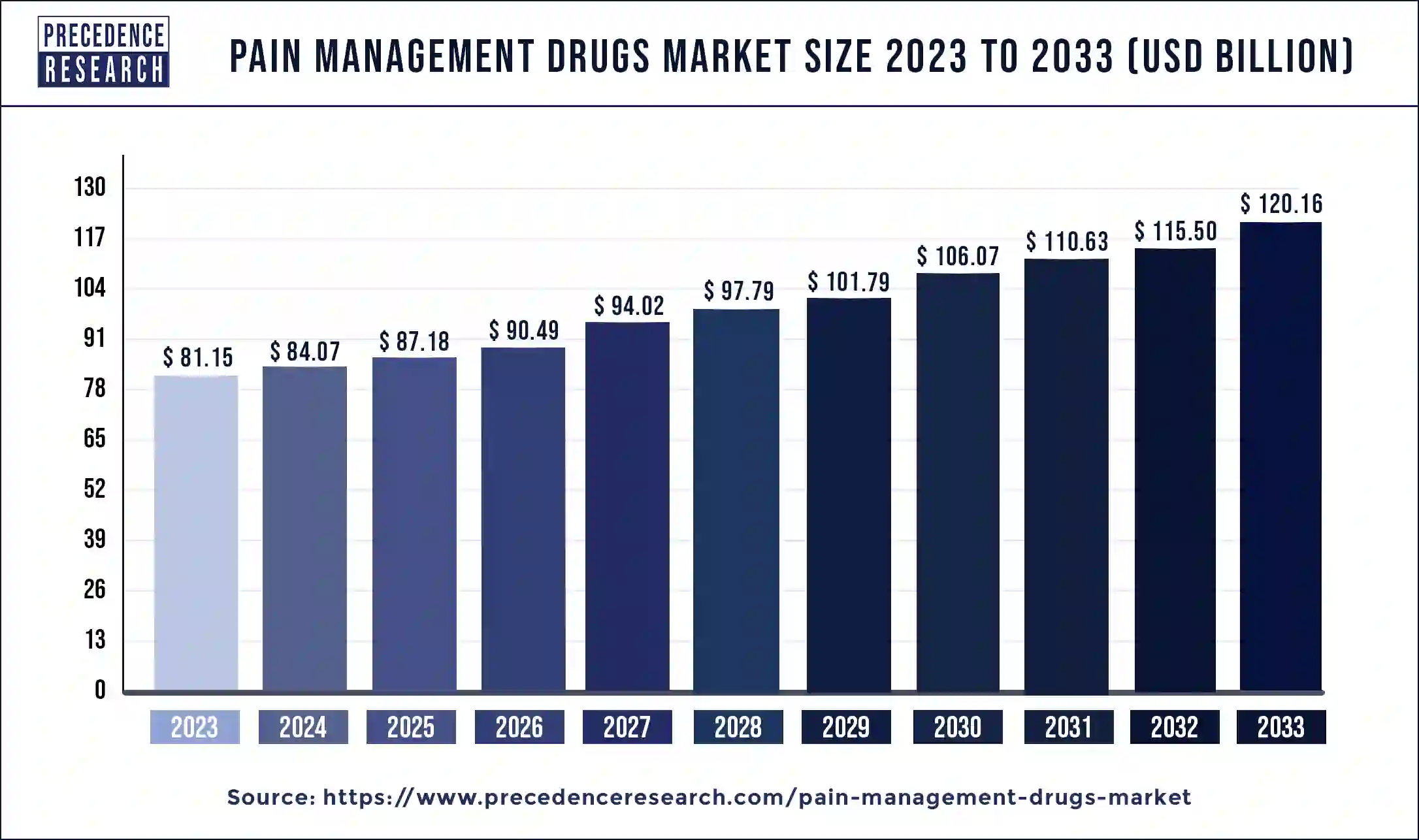
The global pain management drugs market size was estimated at USD 81.15 billion in 2023 and is projected to hit around USD 120.16 billion by 2033, growing at a CAGR of 4% during the forecast period 2024 to 2033.
Pain Management Drugs Market Companies
A New Approach to Opioid Safety
Protega Pharmaceuticals has developed SentryBond technology, which was specifically created to counteract prescription opioid misuse that can culminate into addiction or overdose. This technology has passed rigorous effectiveness testing. ROXYBOND tablets have demonstrated more resistance to manipulation than regular oxycodone IR formulations. Human abuse potential studies also presented the concept of a significantly reduced abuse risk through intranasal and intravenous routes. Other strengths of ROXYBOND have been approved by the FDA since it was previously approved for 5 mg, 15 mg, and 30 mg tablets. This new addition to the 10 mg tablet line provides more flexibility in opioid therapy because it enables physicians to control pain in patients more effectively and safely.
Future Potential for Abuse-Deterrent Medications
Protega Pharmaceuticals has announced the approval of ROXYBOND 10 mg, a pain management medication, as part of its initiatives towards a better prescription opioid overdose management. This is the major development in the fight for safe prescription using SentryBond in developing ROXYBOND, and Protega plans to leverage SentryBond in developing alternative formulations using hydromorphone, hydrocodone, and medications for ADHD. The approval is indurated by Protega's commitment to responsible pain management and addressing prescription drug abuse. The new drug will come out at the end of the current year, thereby increasing flexibility and accuracy for health care providers while improving patients' outcomes. This milestone reflects Protega's commitment to responsible pain management.
We’ve prepared a service to support you. please feel free to contact us at sales@precedenceresearch.com | +1 804 441 9344
April 2025
April 2025
April 2025
April 2025