October 2024
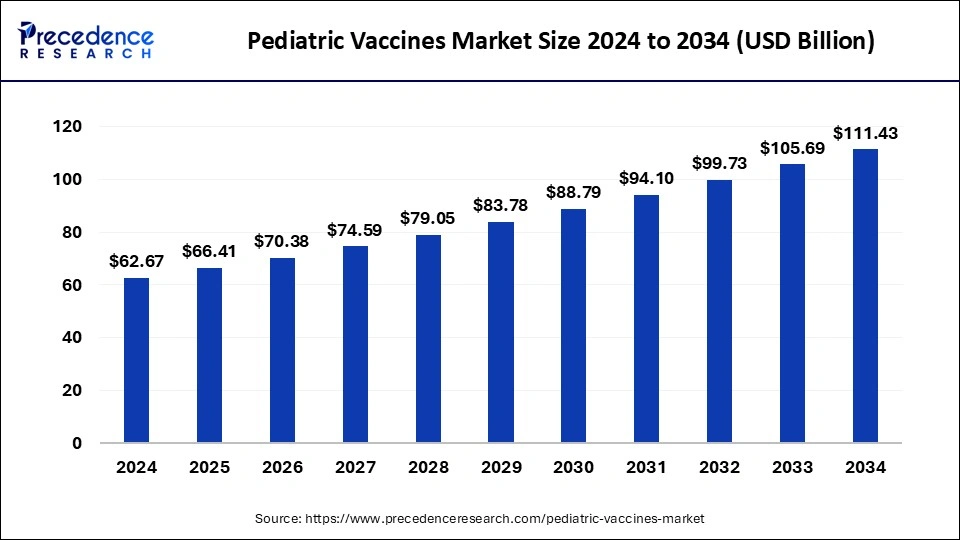
The global pediatric vaccines market size is calculated at USD 66.41 billion in 2025 and is forecasted to reach around USD 111.43 billion by 2034, accelerating at a CAGR of 5.92% from 2025 to 2034. The North America pediatric vaccines market size surpassed USD 23.19 billion in 2024 and is expanding at a CAGR of 5.96% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global pediatric vaccines market size was estimated at USD 62.67 billion in 2024 and is predicted to increase from USD 66.41 billion in 2025 to approximately USD 111.43 billion by 2034, expanding at a CAGR of 5.92% from 2025 to 2034. Vaccines' ability to prevent illness can contribute to the overall health of pediatric populations, which significantly grows the pediatric vaccines market.
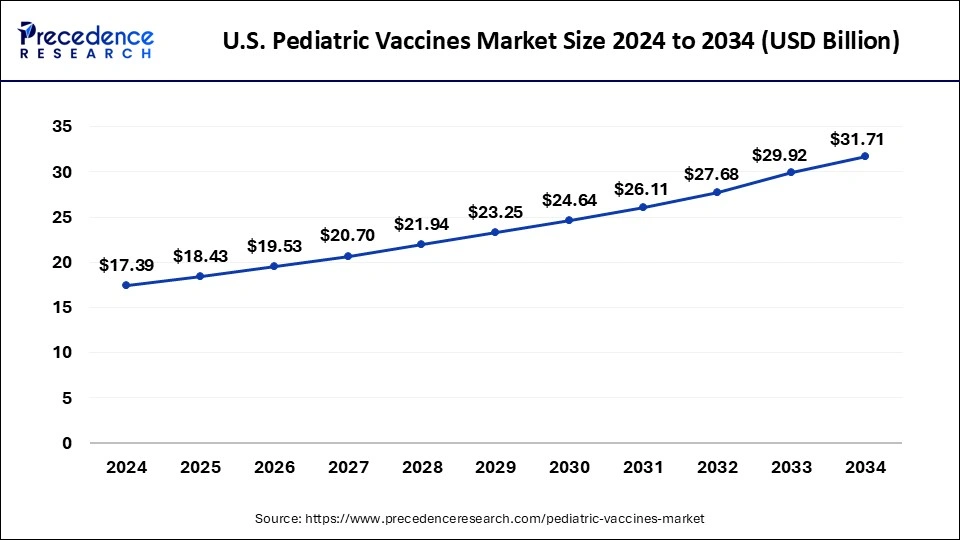
The U.S. pediatric vaccines market size was exhibited at USD 17.39 billion in 2024 and is projected to be worth around USD 31.71 billion by 2034, poised to grow at a CAGR of 6.19% from 2025 to 2034.
North America dominated the pediatric vaccines market in 2024 and is expected to sustain this dominance throughout the forecast period. The economies of North America, and especially the United States, are among the biggest and most dynamic in the world. The demand is driven across a number of industries by this economic strength, which translates into enormous buying power and market size. The area has significant research and development (R&D) skills and is a global leader in technological innovation. This encourages ongoing improvements in infrastructure and technology, especially in industries like IT, healthcare, automotive, and aerospace.
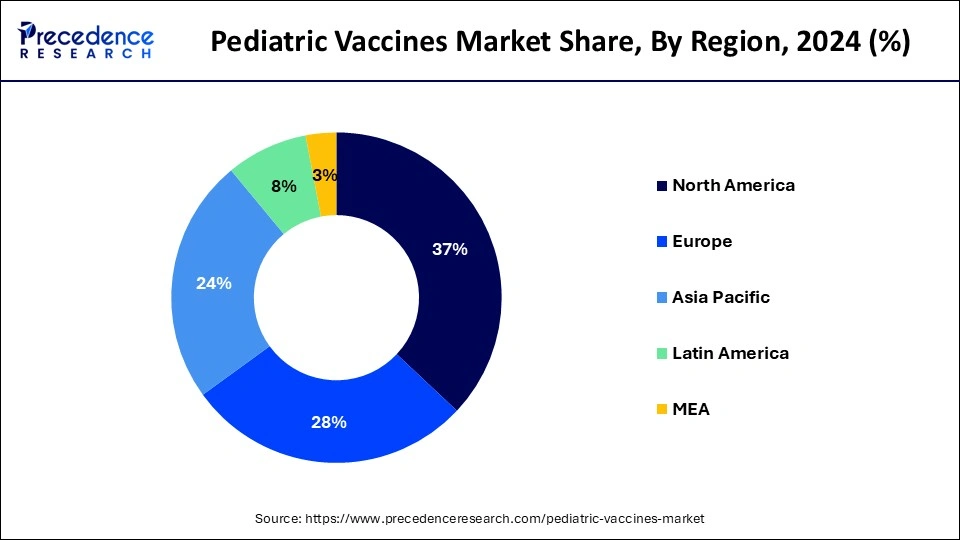
North America boasts a sophisticated infrastructure, encompassing cutting-edge digital infrastructure, utilities, and transportation networks. This makes it easier for products and services to be produced, distributed, and consumed efficiently. The region's competitive advantage is preserved by large investments in R&D across industries. As a result, innovative goods and services are produced, propelling the expansion of the pediatric vaccines market.
The pediatric vaccines market refers to the drugs and vaccines that are used to treat diseases of infants, children, and adolescents from birth to the age of 18. The word pediatric is derived from a Greek word that means pediatric and is ‘healer of children.’ There are some emergency medical drugs and vaccines used in situations of life and death.
The pediatric vaccines market is fragmented with multiple small-scale and large-scale players, such as Serum Institute of India Pvt. Ltd., Sanofi Aventis, CSL Limited, AstraZeneca Plc., Merck & Co., Inc., GlaxoSmithKline Plc., Pfizer Inc., Emergent BioSolutions Inc., Novavax, Inc., Johnson & Johnson.
| Report Coverage | Details |
| Market Size by 2034 | USD 111.43 Billion |
| Market Size in 2025 | USD 66.41 Billion |
| Growth Rate from 2025 to 2034 | CAGR of 5.92% |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Type, Technology, Indication, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Rising prevalence of pediatric diseases
The prevalence of pediatric diseases plays an important role in boosting the pediatric vaccine market. Pediatric diseases are diagnosed in infants, children, and adolescents from birth to the age of 18. The more the increase in the rate of infants, the more the chances of the rising prevalence of pediatric diseases.
Rising awareness of child health
The rising awareness of child health significantly grows the pediatric vaccines market. There are some initiatives that raise awareness of child health. For instance, the National Center for Healthy Safe Children offers resources and technical assistance to states, tribes, territories, and local communities to promote the overall well-being of children, youth, and their families. The rising awareness of child health includes vaccination programs and funding for research and development in pediatric drugs and vaccine manufacturing companies.
Limited medical coverage and healthcare services in low and middle-income countries
The limited medical coverage and healthcare services in low and middle-income countries can negatively affect the pediatric vaccine market. Good medical and healthcare services for a healthy and quality life are an essential element of human rights. Yet more than half of pediatrics can’t get proper medication treatment for pediatric vaccines. So, the limited medical coverage and healthcare services in low and middle-income countries can negatively affect the pediatric vaccines market.
Government and healthcare initiatives
The government and healthcare initiatives can be the opportunity to boost the pediatric vaccines market. Government agencies and healthcare organizations continuously promote strategies to combat infectious diseases in children. This initiative includes vaccination campaigns, educational programs for the parents of children, and efforts to improve healthcare infrastructure.
The monovalent segment held the dominant share of the pediatric vaccines market in 2024. The vaccines that are monovalent, targeting just one pathogen or strain, are easier to create and evaluate than those that are multivalent, targeting several pathogens. As a result, clinical trials and regulatory approval procedures have become less complicated. The monovalent vaccines have the ability to elicit a strong and targeted immune response by targeting just one pathogen. This is especially crucial for pathogens that are highly dangerous to populations of children.
In general, the safety profile of monovalent vaccines is well-established. When compared to vaccines that contain multiple antigens, the risk of adverse reactions is lower with fewer components. Targeted treatment is the best way to manage certain diseases. The introduction of monovalent vaccines, for example, can considerably lower the disease burden in children against a common and dangerous pathogen.
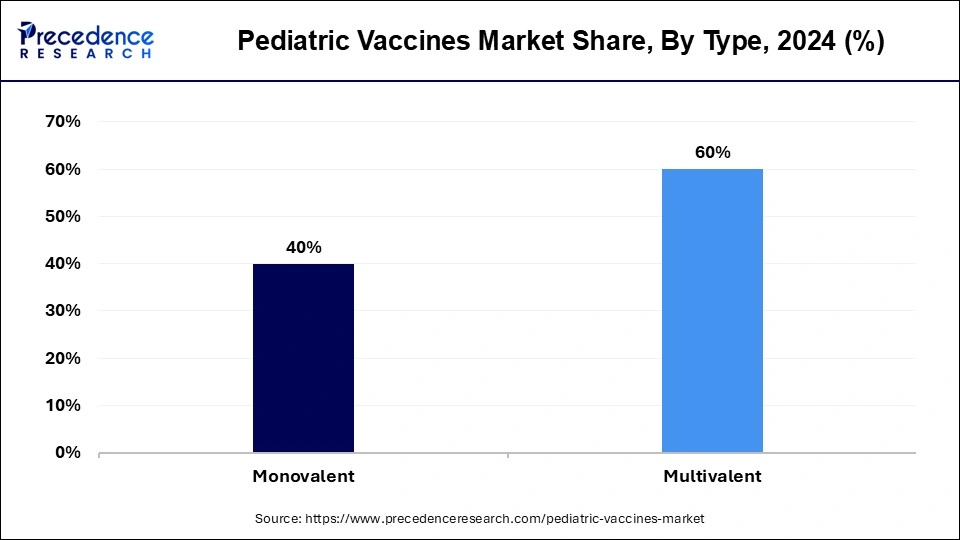
The multivalent segment is expected to expand significantly in the pediatric vaccines market during the forecast period. The number of injections required is decreased by multivalent vaccines, which offer protection against several pathogen strains or varieties in a single dose. For pediatric patients, who frequently need to receive multiple vaccinations, this is especially advantageous. It is easier for parents to follow vaccination recommendations and finish their children's immunization process when multiple vaccines are combined into a single injection. The families benefit from more convenience and a lighter load on healthcare systems when fewer injections are administered, and fewer trips to medical professionals result. This is especially crucial in environments with limited resources.
The subunit, recombinant, polysaccharide, & conjugate vaccines segment led the pediatric vaccines market in 2024. The strong safety profiles of these vaccines are well-known. Comparatively speaking to whole-pathogen vaccines, they lower the risk of adverse reactions by utilizing only particular pathogen components (like proteins or sugars). The vaccines containing subunits, recombinants, polysaccharides, and conjugates are intended to provoke potent and focused immune reactions.
In particular, conjugate vaccines strengthen the immune response, particularly in newborns and early children, by attaching polysaccharide antigens to protein carriers. These vaccines work well against the pathogens that cause serious and prevalent childhood illnesses. Conjugate vaccines, for instance, are used to protect children against the bacteria Haemophilus influenzae type b (Hib) and pneumococcal infections, both of which can be fatal. Because of these vaccines' efficacy and safety, numerous national and international immunization programs give them top priority.
The inactivated vaccines segment is expected to grow at a rapid pace in the pediatric vaccines market during the forecast period. Vaccines that contain dead pathogens or inactivated vaccines have a good safety record. They are especially good for young children whose immune systems are still developing because they do not carry the risk of contracting the disease that they are intended to prevent. These vaccinations effectively protect against a range of diseases by inducing a robust immune response that rarely reverts to a virulent form. The improvements in vaccine technology and production processes have enhanced the efficacy and stability of inactivated vaccines. These developments have increased the likelihood that these vaccinations will be widely used in pediatric populations.
The pneumococcal disease segment dominated the pediatric vaccines market in 2024. The bacterium Streptococcus pneumoniae, which causes pneumococcal disease, can cause pneumonia, meningitis, and sepsis, among other serious illnesses, especially in children. The demand for vaccines is fueled by the fact that parents and healthcare professionals are gravely concerned about them due to their potential severity and frequency. Pneumococcal vaccinations have proven to be very successful in stopping the spread of the bacteria and preventing disease.
The widespread inclusion of pneumococcal vaccines in pediatric vaccination schedules can be attributed in part to their efficacy. The children should receive routine vaccinations against pneumococcal disease, according to national and international health organizations like the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO). Pneumococcal vaccination awareness and uptake are raised by these recommendations.
The influenza segment is expected to grow at the highest CAGR in the pediatric vaccines market during the forecast period. The pediatric vaccine market is anticipated to see growth in the influenza segment as a result of efforts to increase vaccination coverage among pediatric populations in order to prevent the spread of influenza, improve vaccine development, and grow awareness of the significance of flu vaccination for children's health. Further propelling growth in this market will probably be the introduction of more practical and efficient vaccination options brought about by the continuous research and development in the field of pediatric vaccines.

By Type
By Technology
By Indication
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
October 2024
February 2025
August 2024
February 2025