June 2024
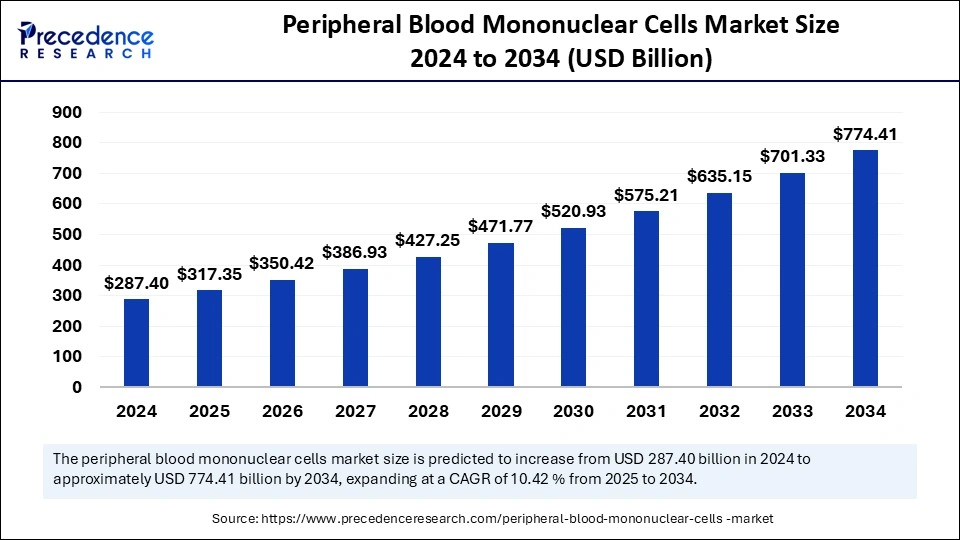
The global peripheral blood mononuclear cells market size is evaluated at USD 317.35 billion in 2025 and is forecasted to hit around USD 774.41 billion by 2034, growing at a CAGR of 10.42% from 2025 to 2034. The North America market size was accounted at USD 129.33 billion in 2024 and is expanding at a CAGR of 10.54% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global peripheral blood mononuclear cells market size was calculated at USD 287.4 billion in 2024 and is predicted to increase from USD 317.35 billion in 2025 to approximately USD 774.41 billion by 2034, expanding at a CAGR of 10.42% from 2025 to 2034. The growth of the peripheral blood mononuclear cells market is attributed to the ongoing research activities for regenerative medicine and the rising development of immunotherapies. The rising demand for cell and gene therapies and a strong emphasis on the utilization of non-animal methods (NAMs) for material safety to expand next-generation risk assessment approaches (NGRA) support market growth.
Artificial intelligence is widely being applied for analyzing peripheral blood mononuclear cells (PBMCs), which is ultimately making processes efficient and providing a more accurate approach compared to conventional methods. AI algorithms and machine learning methodologies are exploited for detecting biomarkers in disease diagnosis by analyzing PBMC profiles for hematological malignancies. AI automates cell differentiation and identification of gene expression patterns, indicating autoimmune diseases or multiple sclerosis by scanning PBMC transcriptomics data.
AI predicts treatment response by analyzing cell images and detects disease-specific metabolic fingerprint markers, allowing diagnosis of autoimmune disorders like systemic lupus erythematosus (SLE) and rheumatoid arthritis. Supervised machine learning algorithms such as random forest, support vector machines (SVM), XGBoost and Artificial Neural Networks (ANN), as well as unsupervised learning methods like clustering algorithms and principal component analysis (PCA), are being integrated with data sources like bulk RNA sequencing (RNA-seq), single-cell RNA sequencing (scRNA-seq), metabolomics and immunophenotyping, further streamlining and enhancing the reliability for PBMC analysis.
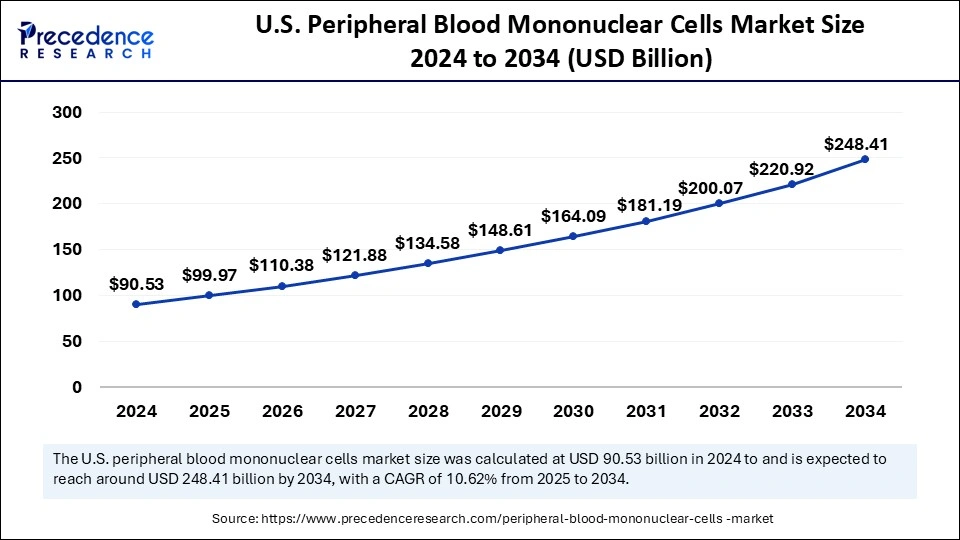
The U.S. peripheral blood mononuclear cells market size was evaluated at USD 90.53 billion in 2024 and is projected to be worth around USD 248.41 billion by 2034, growing at a CAGR of 10.62% from 2025 to 2034.
North America dominated the global peripheral blood mononuclear cells market with the largest share in 2024. The region’s market dominance is driven by the ongoing advancements in cell and gene therapies. There is also a high demand for cell-based therapies. Increased investments in research and development and the presence of advanced infrastructure offering efficient PBMC isolation and manipulation processes for developing therapies further bolstered the market in the region.
U.S. is leading the North American peripheral blood mononuclear cells market. The surging demand for cell therapies, increasing research initiatives for toxicology testing and reaction studies, and expansion of service capabilities by major market players for regional as well as global market outreach contribute to market growth. Moreover, the growing number of Contract Research Organizations (CROs) and rising incidences of chronic and age-related disorders foster market expansion. Government bodies such as the National Institutes of Health (NIH) support the conduction of PBMC-based therapies clinical trials through funding and the U.S. Food and Drug Administration (FDA) regulating the applications of PBMCs in cell therapies for maintaining patient safety and efficacy.
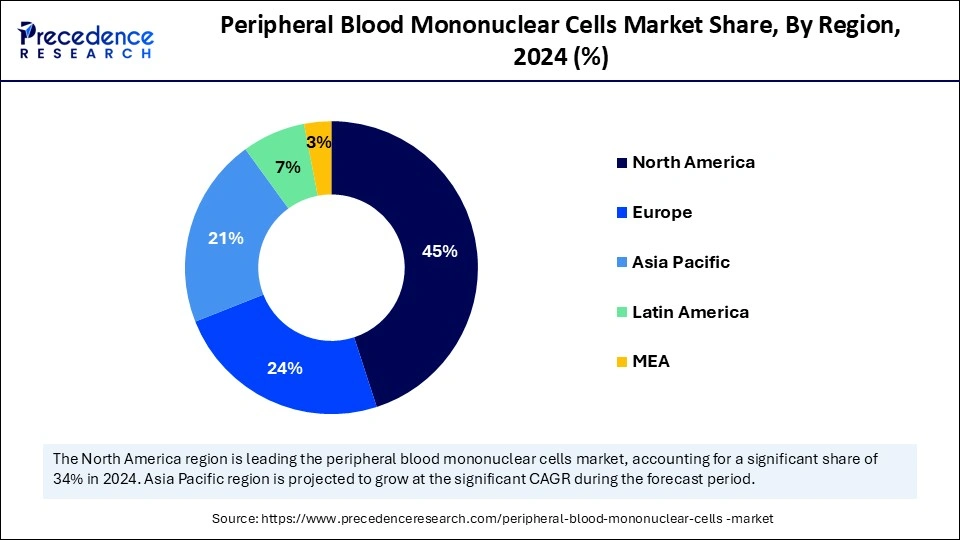
Asia Pacific is expected to witness the fastest growth during the forecast period. The market growth can be attributed to the rising cases of chronic and infectious diseases, large population base, diverse demographics supporting medical advancements through clinical trials, need for personalized medicine, supportive government bodies, and increased expenditure for advancing healthcare infrastructure. China can have a stronghold on the market in Asia Pacific. This is mainly due to the expansion of the biopharmaceutical industry, the presence of a well-established network of newborn screening, which utilizes the PBMC analysis method, and an increasing number of Haemopoietic Stem Cell Transplantation (HSCT) centers.
Europe is projected to grow at a notable rate in the upcoming years. The European peripheral blood mononuclear cells market growth can be attributed to the rising geriatric population, robust research infrastructure, growing demand for cell-based therapies, and ongoing clinical trials. France plays a major role in the market. The rising advancements in biomedical research, increasing demand for disease-specific PBMCs, and the presence of the French National Blood Bank (Etablissement Français du Sang) providing peripheral blood of healthy donors essential for PBMC isolation support market growth.
Peripheral blood mononuclear cells (PBMCs) refer to white blood cells like lymphocytes (T cells, B cells, NK cells), dendritic cells, and monocytes, which have a single, round nucleus. They are key components of the immune system and contribute to defending the body against infections and other dangers. PBMCs are a useful resource tool for drug development and understanding disease mechanisms as they provide a snapshot of an individual’s immune response. The growing incidences of chronic diseases, rising demand for personalized medicine, increasing research initiatives for developing advanced therapies with the use of PBMCs, growing awareness among the population, ongoing clinical studies, development of new facilities with expanded applications, surge in number of outsourcing service providers, and rising investments by governments and several biopharmaceutical companies are contributing to the growth of the peripheral blood mononuclear cells market.
| Report Coverage | Details |
| Market Size by 2034 | USD 774.41 Billion |
| Market Size by 2025 | USD 317.35 Billion |
| Market Size by 2024 | USD 287.4 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 10.42% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product, Application, Techniques, Source, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Burgeoning Applications of PBMC and Rising Demand for Cell Therapies
The potential of cell-based therapies for curing various diseases as well as rare disorders is a key factor boosting their demand. Ongoing technological advancements, increasing investments in developing novel cell therapies with chimeric antigen receptor (CAR)-T cells and CAR-NK cells, and other applications using cell therapy, such as regenerative medicine and immune reconstruction in immunocompromised patients, are driving the market growth. Furthermore, the expanding scope of applications of PBMC drives the growth of the peripheral blood mononuclear cells market. The use of PBMCs is rising in several research applications, such as cell isolation, cell culture, flow cytometry, and cell-based assays. PBMCs find applications in various fields such as toxicology research, drug discovery, immunology, transplantation, and genetics like CRISPR-Cas9 gene editing, autoimmune disorders, metabolic diseases, and hematological malignancies.
Technical Considerations and High Costs
Peripheral blood mononuclear cells (PBMCs) require specialized equipment, skilled professionals, and specific techniques for maintaining the viability of PBMCs during time-consuming isolation processes, storage, and culture, as well as for preventing granulocyte contamination for sustaining the accuracy of downstream experiments. These requirements can restrain market growth due to a lack of technical and infrastructure development.
High costs associated with the isolation and cryopreservation of PBMCs, limited access in some regions or for patient-specific populations, and adherence to regulatory standards are hindering market growth. Additionally, the lack of standardized protocols impeding the comparison of data across different studies and the availability of alternative research and therapeutic applications such as stem cells and other immune cell subsets restrict the growth of the peripheral blood mononuclear cells market.
Expansion of Service Capabilities
Various research organizations and biopharmaceutical companies focus on exploring PBMC applications in specific research areas, understanding customer needs for certain types of PBMCs, analyzing existing PBMC suppliers, and discovering regions with high demands for ensuring structured and reliable PBMC sourcing and processing. Implementation of go-to-market strategies by PBMC manufacturing companies by expanding service capabilities, such as offering high purity and quality of PBMCs to researchers, providing customized PBMC isolation and processing services that are convenient to use, presenting researchers with complete data and technical support, building customer loyalty with direct sales to key researchers and institutions for securing returning business, collaborating with other life science industries for expanding market reach and expertise, are creating opportunities for market growth.
Product Insights
The cryopreserved or frozen PBMC segment dominated the peripheral blood mononuclear cells market with the largest share in 2024. Cryopreservation of PBMCs helps maintain their viability and function, further providing researchers with a convenient and flexible choice for later use, such as in immunological studies and cell-based assays. Companies are developing strategies for optimizing cryopreservation and thawing procedures for the ready-to-use ability of samples after long-term storage while maintaining regulatory adherence. Moreover, the growing demand for PBMCs for various clinical and research applications like antibody development and drug discovery, rising cases of metabolic and autoimmune disorders, and an increasing number of PBMC-based research organizations are supporting segmental growth.
The cultured or fresh PBMC segment is expected to grow at the fastest rate during the forecast period. The growth of this segment can be attributed to the increasing emphasis on developing animal-free testing methods for research purposes and growing awareness of PBMCs’ valuable role in cell and gene therapies for CAR-T cell development, disease research, and drug discovery processes. Furthermore, the surging need for studying immune responses, increased demand for PBMCs for R&D activities, development of comprehensive quality management programs for monitoring PBMC processing as well as several applications of cultured PBMCs such as for developing new vaccine candidates and in recurrent implantation failure (RIF) therapy for improving live birth rates are the factors expected to drive the market growth of this segment in the upcoming years.
The immunology segment held the largest share of the peripheral blood mononuclear cells market in 2024. This is mainly due to the increased adoption of PBMCs in immunology research for studying immune cell behavior and disease progression and factors influencing long-term immunity due to their ability to both innate and adaptive immune responses. In immunology, there is a strong focus on using PBMCs as a liquid biopsy for predicting treatment response, comprehensive immunophenotyping with flow cytometry, monitoring immune responses, and in systems biology and bioinformatics approaches such as epigenomics, transcriptomics, and metabolomics for identifying disease markers and their mechanisms. PBMCs are also applied in cancer immunology for tumor microenvironment (TME) characterization, immunotherapy response prediction, and co-culturing PBMCs with organoids, as well as other applications such as for engineering immune cells with genome editing technologies for assessing immunogenicity of viral proteins through vitro models and in metabolic reprogramming of PBMCs during immune responses.
The infectious disease segment is anticipated to witness the fastest growth over the forecast period. PBMCs are widely applied in the research of infectious diseases, such as HIV, influenza virus infection, filarial infections, and SARS-CoV-2, and also for studying the response of fungal infections caused by Cryptococcus neoformans and Candida albicans. The growth of this segment can be linked to a growing infectious disease burden, surging outbreaks of drug-resistant infections, rising investments in infectious disease research and development, patient stratification for personalized treatment strategies, ongoing advancements in diagnostic technologies, and increased awareness among the population about early diagnosis and disease prevention.
The density gradient centrifugation segment accounted for the largest share of the peripheral blood mononuclear cells market in 2024. The density gradient centrifugation technique is applied for separating particles based on their densities with a gradient of differing densities, further isolating specific components such as cells, viruses, or macromolecules and can be supplemented with cell separation methods like buoyancy-activated cell sorting (BACS) or fluorescence-activated cell sorting (FACS) in centrifugation for enhancing the efficacy of processes. The low costs of density gradient media, easy installation in any lab setting, and no requirement of specialized equipment bolstered segmental growth.
Furthermore, the growing demand for cell separation and ongoing progress in cell-based therapies and diagnostic methods contributed to segmental growth. The increased use of centrifugation processes for research activities and the need for developing innovative and effective products and treatments for the rising chronic disease burden are factors boosting the growth of this segment.
The leukapheresis segment is anticipated to show the fastest growth during the predicted timeframe. The Leukapheresis process refers to the separation of white blood cells from a blood sample, providing highly purified product (Leukopak) with a rich concentration of PBMCs compared to buffy coat isolations. Various advantages of the Leukapheresis process, such as reduced contamination, minimized donor variability, efficiency in cell isolation, versatile applications, production of autologous therapies, and improved PBMC yield, are expected to drive the growth of this segment in the upcoming years.
The human segment dominated the peripheral blood mononuclear cells market with the largest share in 2024. Human-sourced PBMCs are readily obtained by laboratories with non-invasive methods such as simple blood draws or blood products such as leukopaks and Leukocyte Reduction System (LRS) cones and primary cell suppliers for isolation. The surge in demand for cell therapies, the use of PBMCs in toxicology testing for assessing the efficacy and safety of drugs, increased emphasis on immunology and vaccine development, as well as the use of PBMCs as a convenient and accessible source of cells for induced pluripotent stem cells (hiPSCs) reprogramming and protein-based biomarkers bolstered the growth of this segment.
The animal segment is anticipated to grow at a remarkable CAGR in the coming years. Animal-sourced peripheral blood mononuclear cells (PBMCs) act as a valuable tool, offering several advantages in research. They have potential therapeutic applications due to the easy isolation process with minimally invasive methods, allowing repeated sampling for longitudinal studies and their immunomodulatory properties and low immunogenicity, especially in species like horses. Equine PBMCs are widely used for immune function assessment. They are also used in research for studying the immune system of horses and in xenotransplantation studies. They are a source of multipotent mesenchymal stromal cells (MSCs), which can be differentiated into several cell types like adipocytes, chondrocytes, and osteocytes applied for the treatment of musculoskeletal injuries.
By Product
By Application
By Techniques
By Source
By Region
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
June 2024
January 2025
August 2024
March 2024