December 2024
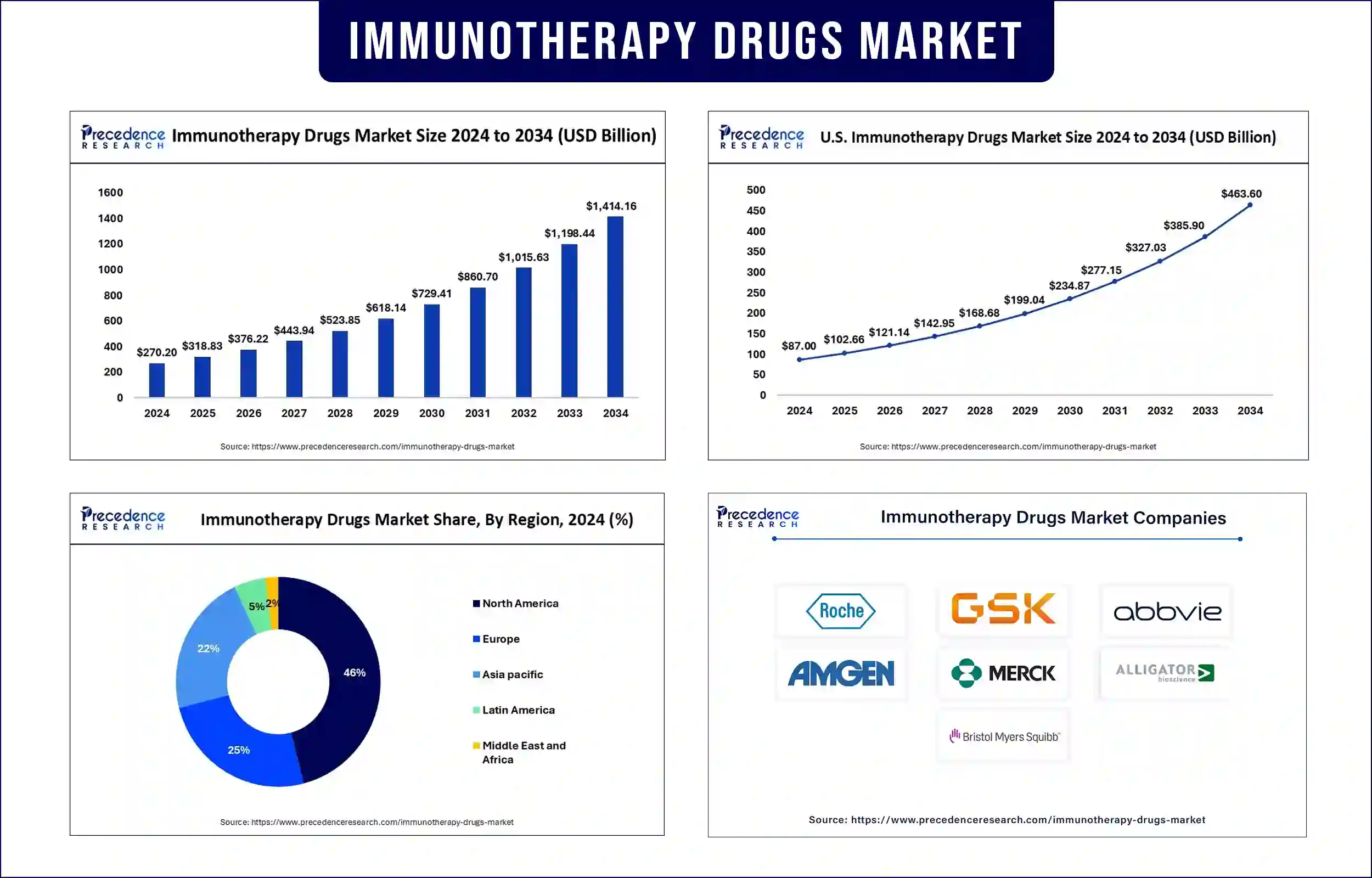
The global immunotherapy drugs market revenue was valued at USD 318.83 billion in 2025 and is expected to attain around USD 1198.44 billion by 2033, growing at a CAGR of 18% during forecast period. The increasing prevalence of chronic diseases, advancements in monoclonal antibodies, and growing investment in personalized medicine are driving market growth.
The immunotherapy drugs market identifies research opportunities to create biologic-based and targeted therapeutic remedies that boost immune responses against cancers and immune disorders, and infectious diseases. More people choose immunotherapy now for its extended treatment results along with minimum adverse effects relative to traditional medical approaches.
The market experiences expansion due to the rising occurrence of persistent illnesses and developments in monoclonal antibody technology, along with increasing support for customized medical therapies. The immunotherapy drugs market demonstrates continuous transformation, as the regulatory backing along with technological breakthroughs and clinical need filling efforts.
Rising Adoption of Personalized Medicine
Advances in genomics and biomarker research are enabling the development of personalized immunotherapies, improving treatment outcomes, and reducing adverse effects. During genomic data analysis, artificial intelligence (AI) has enhanced the discovery of biomarkers connected with immunotherapy reactions, which helps develop custom treatment approaches.
The development of personalized cancer vaccines that specifically target tumor neo-antigens demonstrates encouraging clinical trial results that create new possibilities for immunotherapy success. Genomic research, together with biomarker developments, plays a fundamental part in advancing personalized medicine through the advancement of exact immunotherapeutic treatments.
Increased FDA and EMA Approvals
Regulatory bodies are fast-tracking approvals for innovative immunotherapies, such as PD-1/PD-L1 inhibitors, expanding treatment options for various diseases. The approval period of combination immunotherapies is receiving increased attention from regulatory agencies, which leads to shorter approval times to satisfy unmet medical requirements. Furthermore, the improved patient access to leading-edge immunotherapies strengthens these treatments as essential components of current therapeutic methods.
The U.S. Food and Drug Administration (FDA) authorized accelerated approval for new immunotherapies during 2024 while giving sanction to next-generation checkpoint inhibitors that enhance immune activation. Immunotherapy-based treatment approvals at the European Medicines Agency (EMA) have become efficient for both rare and aggressive forms of cancer. The Project Orbis program run by the FDA enables the concurrent global assessment of cancer medications, which speeds up international patients' access to vital immunotherapy treatments.
Technological Advancements in CAR-T Cell Therapy
Ongoing research in CAR-T cell therapy is enhancing its effectiveness in hematologic cancers and expanding its potential into solid tumors. The U.S. Food and Drug Administration (FDA) issued approvals for new CAR-T therapies directed toward treating resistant blood cancers during 2024, which enhanced patient survival statistics. Scientists are creating modern CAR-T cell versions that demonstrate longer survival times and lower damage to human tissue, and improved cancer cell detection properties.
Circular DNA sequences from editing technologies, including CRISPR, help engineers optimize CAR-T cell programming to improve treatment results. Scientists conduct clinical trials of allogeneic (off-the-shelf) CAR-T therapies as they seek to achieve cost reduction and wider availability beyond the traditional protocol of using autologous cells in standard therapies. The advancement of CAR-T therapies extends their application to treat solid tumors, which represents a key development in immune system cancer treatment.
Expansion into Autoimmune and Infectious Diseases
Immunotherapy is increasingly being explored beyond oncology, with new drugs targeting autoimmune diseases and emerging infectious diseases, including COVID-19. In 2024, scientists discovered promising monoclonal antibodies that showed potential for treating rheumatoid arthritis, lupus, and multiple sclerosis by combating inflammation and managing immune responses. The FDA and EMA dedicate their efforts to reviewing cytokine therapies and checkpoint inhibitors for autoimmune conditions, which accelerates upcoming drug releases.
Scientists are currently developing T-cell-based immunotherapies to tackle HIV and hepatitis B chronic viral infections through clinical studies that demonstrate promising results in removing viral reservoirs from patients' bodies. Researchers expect that pharmaceutical industries developing immunotherapy applications will lead to transformations in disease area treatments.
North America holds a dominant position in the immunotherapy drugs market, driven by strong R&D investments, a high prevalence of chronic diseases, and the presence of major pharmaceutical companies. The FDA, through its expedited approval processes, including accelerated approval and priority review, lets innovative therapies with serious therapeutic potential reach the market rapidly.
A 2024 NIH Notice of Special Interest (NOSI) served to promote scientific investigations about the fundamental mechanisms behind adverse immune reactions that occur during cancer immunotherapy while demonstrating dedication to controlling such side effects. Protecting lives becomes faster due to strong life-saving infrastructure, which enables efficient innovation and quicker accessibility of therapeutic drugs to patients.
Asia Pacific is anticipated to experience the fastest growth in the immunotherapy drugs market, attributed to rising cancer incidence, increasing healthcare expenditures, and expanding biopharmaceutical manufacturing capacities in countries like China, Japan, and India. The pharmaceutical manufacturing sectors of China, together with other countries, have received significant investments to enhance their production capacity of vital biologic therapies.
WHO's strategy to make biologic copies accessible has delivered additional strength to biopharmaceutical production centers throughout the middle-income areas of the region. The increased healthcare expenditures and recent developments lead to greater access and acceptance of immunotherapy treatments throughout Asia Pacific, thus improving cancer treatment results and managing the escalating cancer conditions.
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 318.83 Billion |
| Market Revenue by 2033 | USD 1,198.44 Billion |
| CAGR | 18% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
By Drug Type
By Therapeutic Area
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/1472
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com|+1 804 441 9344
December 2024
January 2025
April 2025
January 2025