January 2025
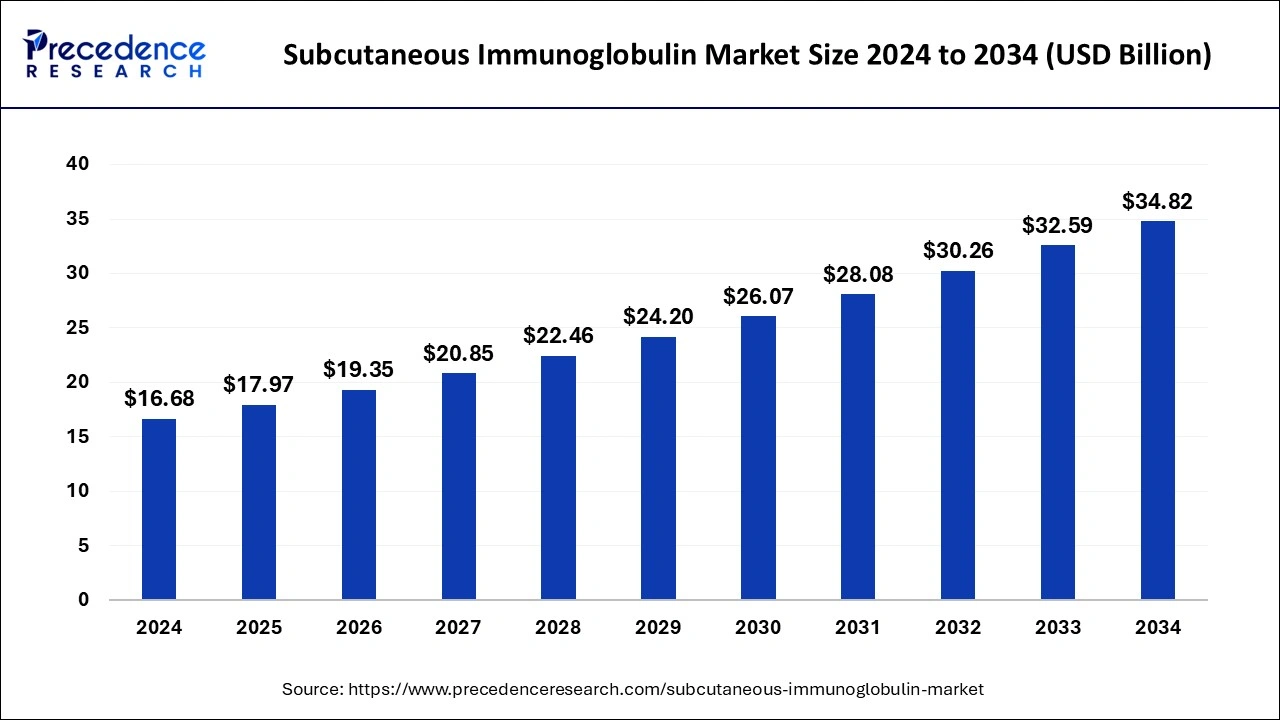
The global subcutaneous immunoglobulin market size is calculated at USD 17.97 billion in 2025 and is forecasted to reach around USD 34.82 billion by 2034, accelerating at a CAGR of 7.64% from 2025 to 2034. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global subcutaneous immunoglobulin market size was estimated at USD 16.68 billion in 2024 and is predicted to increase from USD 17.97 billion in 2025 to approximately USD 34.82 billion by 2034, expanding at a CAGR of 7.64% from 2025 to 2034.
The global subcutaneous immunoglobulin market offers a method of administering immunoglobulin (Ig) therapy by injecting immunoglobulin beneath the skin (subcutaneously). Immunoglobulins are proteins that play a crucial role in the immune system, helping to fight off infections and providing immunity. SCIg therapy is often used in the treatment of certain immunodeficiency disorders, where the body's immune system is unable to produce enough antibodies to effectively combat infections.
By delivering immunoglobulins subcutaneously, the therapy helps supplement the deficient antibodies, boosting the individual's immune response. Compared to intravenous immunoglobulin (IVIg) administration, which involves injecting immunoglobulins directly into a vein, SCIg allows for a slower and more sustained absorption of the immunoglobulins into the bloodstream.
This method is often preferred by patients because it can be administered at home, reducing the need for frequent hospital visits. The choice between subcutaneous and intravenous administration depends on various factors, including the patient's condition, preferences, and the specific requirements of the immunoglobulin therapy.
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 7.64% |
| Market Size in 2025 | USD 17.97 Billion |
| Market Size by 2034 | USD 34.82 Billion |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | By Product Type, By Application, and By End-use |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
An increase in immune-related diseases
As immunodeficiency conditions become more prevalent, the subcutaneous immunoglobulin market is growing. The need is being driven by conditions like primary and secondary immunodeficiency diseases. Because patients with compromised immune systems require effective subcutaneous immunoglobulin inoculations for immune system augmentation, the subcutaneous immunoglobulin market is growing. Due to its simplicity as compared to traditional intravenous methods, the market is expanding. As awareness of immune-deficiency diseases and improved diagnostics increase, the market will likely see a rise in demand for affordable and effective immunoglobulin treatments.
Advantages of administration
Ongoing research and development efforts have led to the development of advanced SCIG formulations with improved stability, bioavailability, and pharmacokinetic profiles. Newer SCIG products offer longer dosing intervals, reduced infusion volumes, and enhanced tolerability, which contribute to improved patient compliance and treatment outcomes. SCIG offers several advantages over intravenous immunoglobulin (IVIG) therapy, including convenience, flexibility, and reduced healthcare resource utilization. Patients can self-administer SCIG at home, eliminating the need for frequent hospital visits and intravenous infusions, which enhances patient convenience and quality of life.
High cost and limited awareness
The cost of SCIg therapy, including the immunoglobulin product itself and associated administration supplies, can be a significant factor limiting its widespread adoption. Affordability and reimbursement policies may impact patient access to SCIg treatment. Moreover, a lack of awareness among healthcare providers and patients about the benefits of SCIg therapy compared to other forms of immunoglobulin administration, such as intravenous immunoglobulin (IVIg), could be a constraint.
Technological advancements
Significant subcutaneous immunoglobulin market expansion is being driven by technological advancements. Drug delivery innovations, such as the use of autoinjectors and vaccination devices, improve behavior and increase patient comfort and compliance. Production processes yield incredibly complex and sophisticated formulas, optimizing the results of therapy. Advancements in diagnosis and observation methods permit personalized treatment regimens and simultaneous tracking of immunoglobulin levels. The subcutaneous immunoglobulin market is changing as a result of the evolving technical landscape, which promises improved patient outcomes, availability, and productivity in the treatment of immunodeficiency diseases.
The IgG segment held the largest share of the subcutaneous immunoglobulin market in 2024. IgG is a type of immunoglobulin, specifically one of the four subclasses of antibodies found in the blood. Immunoglobulin therapy involves the administration of immunoglobulin products, which can include IgG, to individuals with primary immunodeficiency disorders or certain autoimmune conditions. The increasing product launches are expected to propel the segment expansion.
IgM is one of the five major classes of immunoglobulins, or antibodies, found in the blood. Immunoglobulin therapy typically involves the replacement or supplementation of immunoglobulins, including IgM, for individuals with primary immunodeficiency disorders or certain autoimmune conditions. Immunodeficiency disorders can result in a lack of one or more immunoglobulin classes, including IgM. Replacement therapy aims to provide the deficient antibodies, and the composition of immunoglobulin products may include IgM. Thereby, driving the segment expansion.
The primary immunodeficiency segment dominated the subcutaneous immunoglobulin market. Immunoglobulin surface is used subcutaneously as a patient's primary immunodeficiency treatment, transforming healing gait. The need for subcutaneous immunoglobulin is increasing because of the approval of home spun management, which gives patients self-managing options, reduces reliance on hospital visits, and promotes dependability. Its adaptable treatment plan with customized dosage regimens accommodates various lifestyle choices.
Immunoglobulin administered subcutaneously ensures a constant supply of immunoglobulins, maintaining stable antibody levels and protecting against contaminations. It increases patient satisfaction with the least amount of overall consequences, offering a recommended and feasible solution. Thus, this is expected to drive the global subcutaneous immunoglobulin market growth.
The hospital segment held the largest share of the subcutaneous immunoglobin market. The segment is observed to sustain the growth during the forecast period. While subcutaneous immunoglobulin therapy is often associated with home-based treatment due to its convenience and self-administration potential, hospitals also play a role in the administration of SCIg. Some patients may receive their SCIg therapy within a hospital setting, particularly if they require medical supervision or if they are newly diagnosed and need initial training.
Hospitals admit patients for immunoglobulin therapy, including SCIg, for various reasons. This could include patients with primary immunodeficiency disorders or other conditions where immunoglobulin replacement is indicated. In such cases, hospitals provide the infrastructure and expertise to administer the therapy.
The homecare segment is expected to grow at the highest CAGR during the forecast period. One of the primary drivers for the growth of the homecare segment in the market is the convenience it offers to patients. Home-based administration allows individuals to self-administer their SCIg therapy, reducing the need for frequent hospital or clinic visits. Additionally, the development of user-friendly and portable infusion devices has facilitated the shift toward home-based SCIg administration. Advances in technology have made it easier for patients to self-administer their treatments. Thereby, driving the segment expansion.
North America, in 2024, held the largest share of the subcutaneous immunoglobulin market because of advances in technology that have improved productivity in prescription and delivery processes while raising awareness among patients and healthcare donors, boosting the market. Growing healthcare spending is correlated with the recognition of its clinical utility and the approval of the government to expand treatment alternatives. Patient-centered care promotes the ease of SCIG, which promotes wider adoption. The aggressive topography consists of stimulating inventions from both surfacing and entrenched actors. Collaboration drives the gathering of facts, insurance coverage influences strategy, and the COVID-19 effect emphasizes the sector's viability by highlighting its critical role in immune-related scenarios.
Besides, the Asia Pacific is expected to grow at the highest CAGR during the forecast period. Increasing awareness among healthcare professionals and patients about the benefits of SCIg therapy has contributed to its adoption. Education programs and awareness campaigns have played a role in informing the medical community and the public. Moreover, government initiatives and policies supporting the treatment of immunodeficiency disorders may impact the availability and affordability of SCIg in the region. These policies can include reimbursement mechanisms and incentives. Thus, this is expected to drive the subcutaneous immunoglobulin industry in the region.
By Product Type
By Application
By End-use
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
January 2025
July 2024
October 2024
April 2024