October 2024
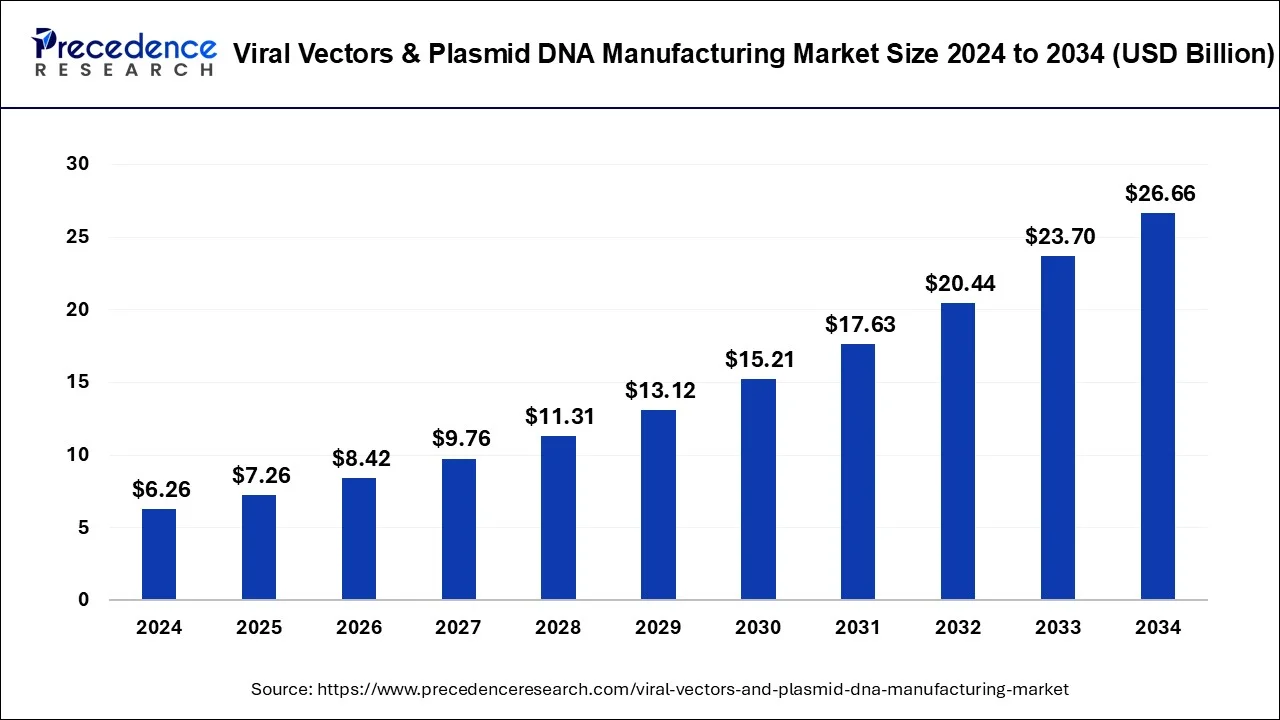
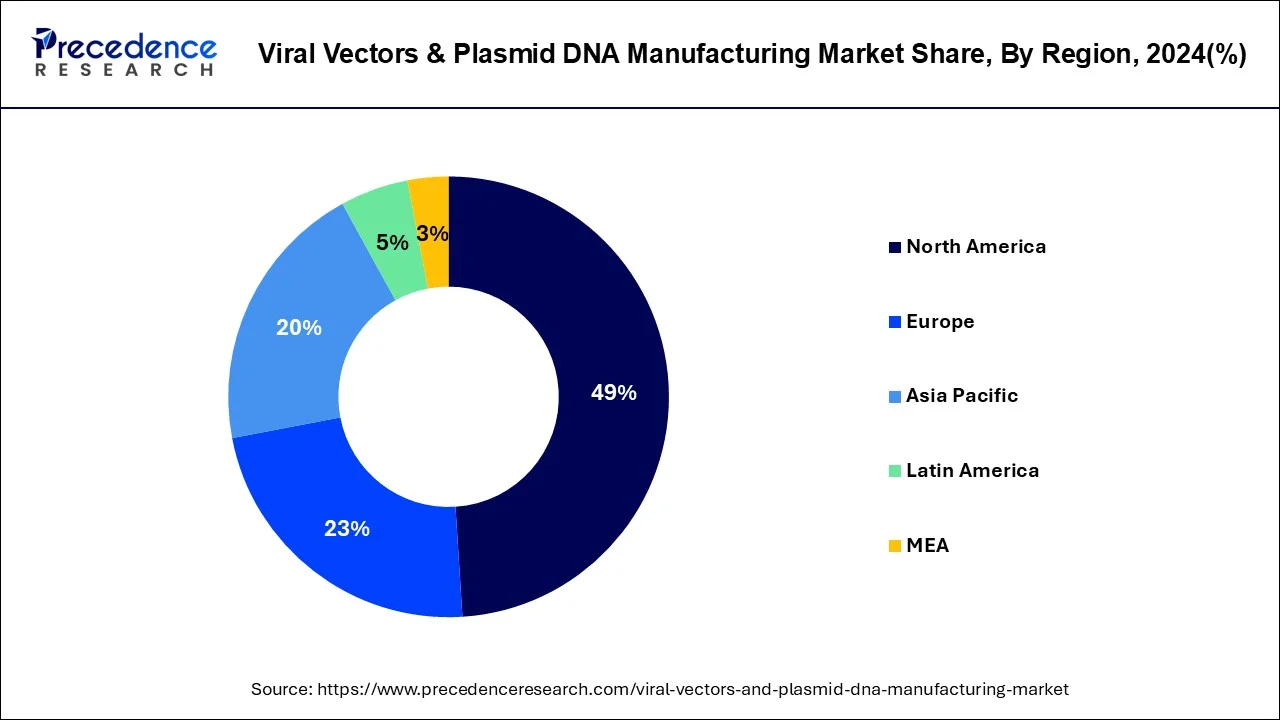
The global viral vectors & plasmid DNA manufacturing market size is calculated at USD 7.26 billion in 2025 and is forecasted to reach around USD 26.66 billion by 2034, accelerating at a CAGR of 15.59% from 2025 to 2034. The North America viral vectors & plasmid DNA manufacturing market size surpassed USD 3.07 billion in 2024 and is expanding at a CAGR of 15.60% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global viral vectors & plasmid DNA manufacturing market size was calculated at USD 6.26 billion in 2024 and is projected to grow around USD 26.66 billion by 2034, growing at a CAGR of 15.59% from 2025 to 2034. The viral vectors & plasmid DNA manufacturing market is driven by the increasing adoption of gene therapies for rare and genetic diseases.
The creation of vaccines, biotechnology, and gene therapy all depend on viral vector engineering. Gene therapies aim to replace or fix defective genes in a person's cells through genetic engineering, offering the possibility of one-dose treatments for various illnesses.
AI algorithms can detect putative gene targets and confirm their therapeutic significance by analyzing extensive molecular and genomic information. This enables the identification of new gene candidates and the evaluation of their potential for gene therapy treatments. By combining several datasets, AI is excellent at predicting the safety and effectiveness of gene therapy. It improves patient responses using prediction models to evaluate treatment results and streamline procedures.
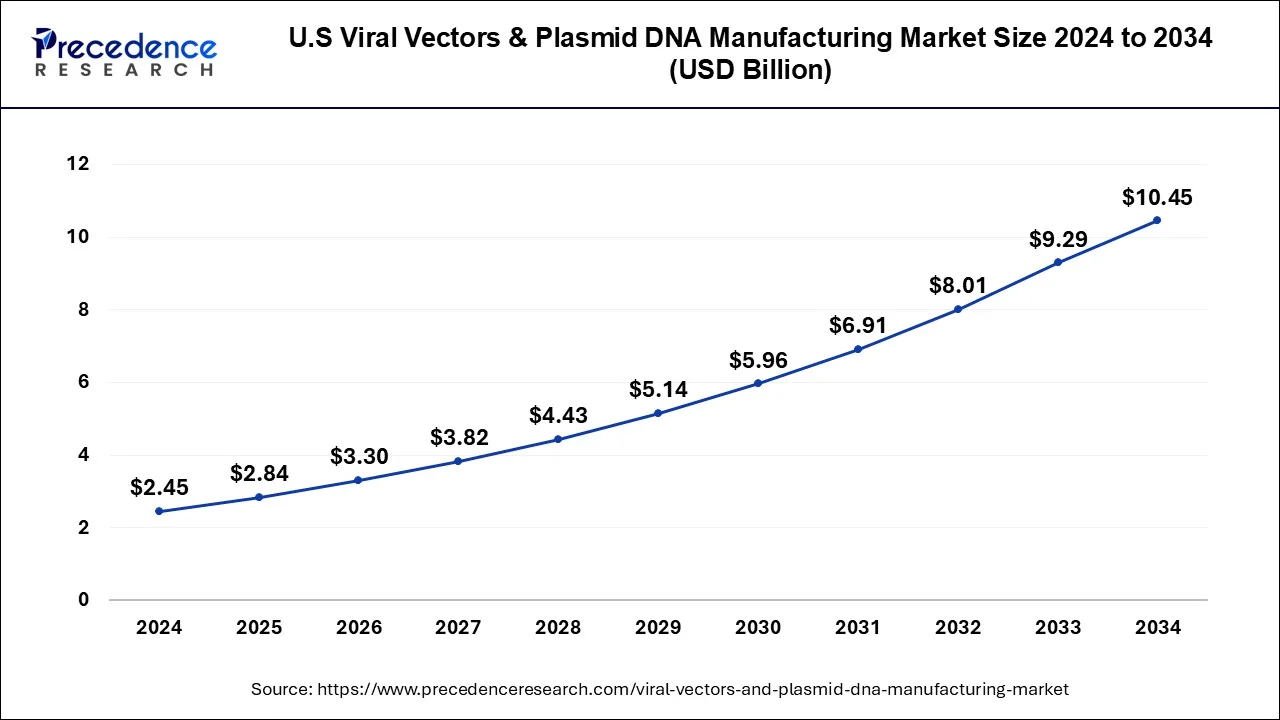
The U.S. viral vectors & plasmid DNA manufacturing market size was valued at USD 2.45 billion in 2024 and is projected to reach around USD 10.45 billion by 2034 with a CAGR of 15.61% from 2025 to 2034.
Current FDA approval of advanced therapies such as Yescarta and Kymriah (tisagenlecleucel) supports the major investment in vector manufacturing market in U.S. Also, industrialization of gene therapy manufacturing and clinical transformation is predictable to persuade noteworthy progress across several Asian nations. In order to speed up vector manufacturing in Asia Pacific many multinational companies are cooperating with Asian based companies. At present, U.S. and Europe have appeared as vector manufacturing hubs in spite of the fact that the first 3 gene therapy candidates (Oncorine®, Gendicine® andRexin-G®) were permitted in Asian countries. This success is attributable to high volume of enduring clinical studies in these established regions. Around 68% of the total international active clinical studies for gene therapies are proceeding in North America. The second foremost market for trial is Europe where about 21% of the trials are under progress.
United States
Growing occurrence of target disorders and diseases and efficiency of viral vectors in gene therapy delivery are driving the growth of viral vectors & plasmid DNA manufacturing market. Constant research into viral vector-based gene & cell therapies and availability of funding for gene therapy improvement are further supporting this growth. The complication of viral vectors is much larger than traditional biologics. As a consequence, manifold orthogonal methods are implemented to comprehend the quality and physicochemical properties of viral vector products. This multifaceted methodology will endure unless revolutionary technologies are developed that permit the integration of the results from numerous analyses.
Rising interest in vector manufacturing area is emphasized by the growing number of partnerships or collaborations amongst the various organizations involved in this sector. The intentions behind these partnerships vary for different purposes. Collaborations have been signed for purposes including production of vector promoters and development or acquisition of manufacturing facilities and out / in licensing of vector manufacturing technology among others.
However, some of the factors limiting the growth of this market include, “noise” during process development.This noise in cell-based assays may offer challenges for the evaluation of process improvements. In order to overcome this trouble, trending is accomplished to develop confidence that an improvement has been achieved.
| Report Highlights | Details |
| Market Size by 2034 | USD 26.66 billion |
| Market Size in 2024 | USD 6.26 billion |
| Growth Rate | CAGR of 15.59% From 2025 to 2034 |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Vector Type, Application, Workflow, End-User, Disease |
| Regional Scope | Asia Pacific, North America, Europe, Latin America, Middle East and Africa |
This research study segments global market based on various parameters such as type, application, disease, and end user. Depending on type, this market is classified into adenovirus, plasmid DNA, lentivirus, retrovirus, AAV, and other viral vectors. In 2024, AAV dominated the market with around 21% share in terms of revenue of the total market. The adeno-associated viral vector manufacturing segment is estimated to grow at the utmost CAGR throughout the forecast period due to their applications in most of the cell-based gene therapies. Integration ability with large transgenes and simple production at high titers are some of the significant facts that help to occupy considerable share of this vector in the overall market.
However, lentiviral vectors are anticipated to generate high revenue during years to come on account of the major accomplishment of the lentiviral-based gene therapy product in 2023.
Downstream processing emerged as dominating workflow segmentation account of its highly complex procedures conducted for purification and polishing of clinical-grade final products. Further, the costly purification techniques have led to the great revenue generation of this segment compared with upstream processing segment.
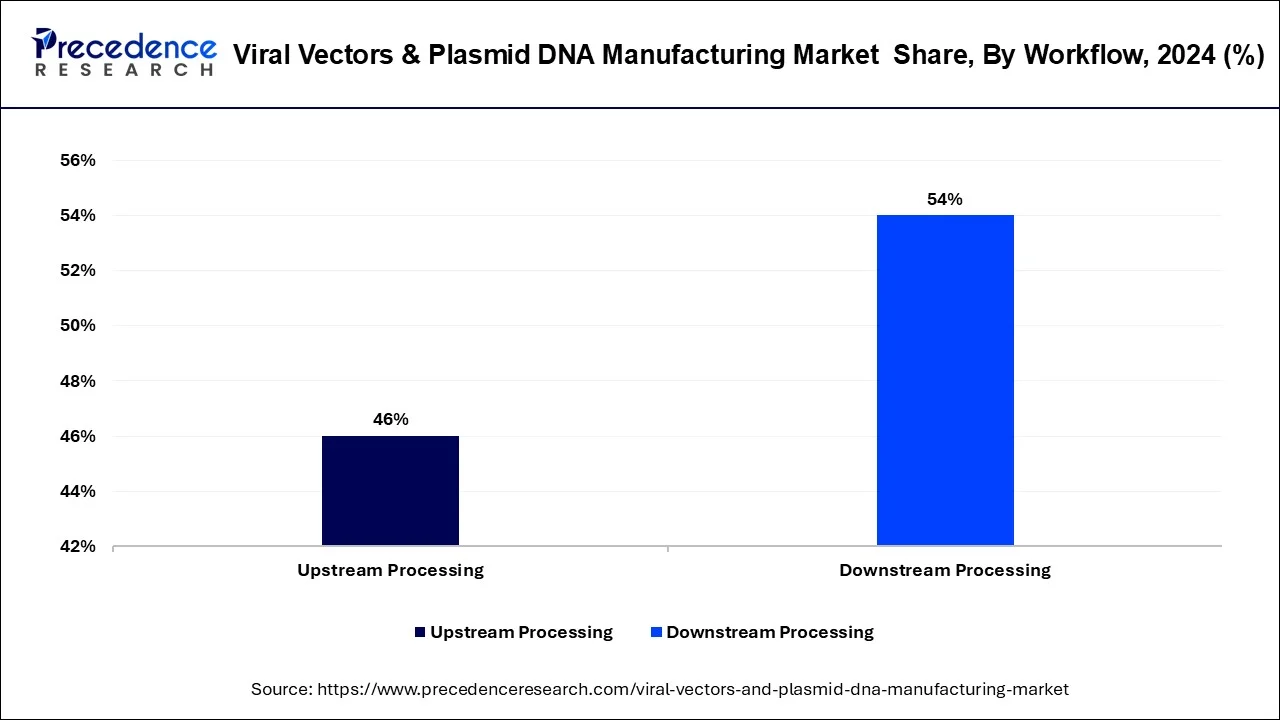
In 2023, downstream processing dominated the market in terms of revenue of the total market. Downstream processing is projected to grow at highest CAGR during forecast period. Downstream processing reported for the major share due to highly complex procedures conducted for polishing and purification of clinical-grade final products. Furthermore, the high cost of purification techniques has led to the high revenue generation in this segment.
Application segment of this viral vectors & plasmid DNA market including gene therapies and antisense and RNAi, emerged as dominating vectors in terms of overall revenue share. At present, small interfering RNAs (siRNAs) are assumed as substantial tools for post-transcriptional gene silencing through a genetic analysis of cells. The existence of pipeline products is estimated to liftthe growth of this sector during years to come.
In 2023, gene therapy dominated the market in terms of revenue of the total market.
Depending on end-users the global viral vectors & plasmid DNA manufacturing market is categorized into biopharmaceutical and pharmaceutical companies and research institutes. In 2024, pharmaceutical and biopharmaceutical companies are projected to occupy prime share of the global vector market. This is on account of the effective launch of viral vector gene therapies and a vigorous pipeline of such therapies that leads growth of the pharmaceutical and biopharmaceutical companies in coming years. Further, constant introduction of innovative therapies plus consequent upsurge in the number of gene therapy-based discovery programs by firms boost this growth. The number of biotech companies implementing vectors for therapeutics production is further projected to grow during near future.
Different kinds of diseases analyzed in this research study include genetic disorders, cancer, infectious diseases, and other diseases. In 2023, cancer dominated the market in terms of revenue of the total market. Cancer is also projected to grow at highest CAGR during forecast period.
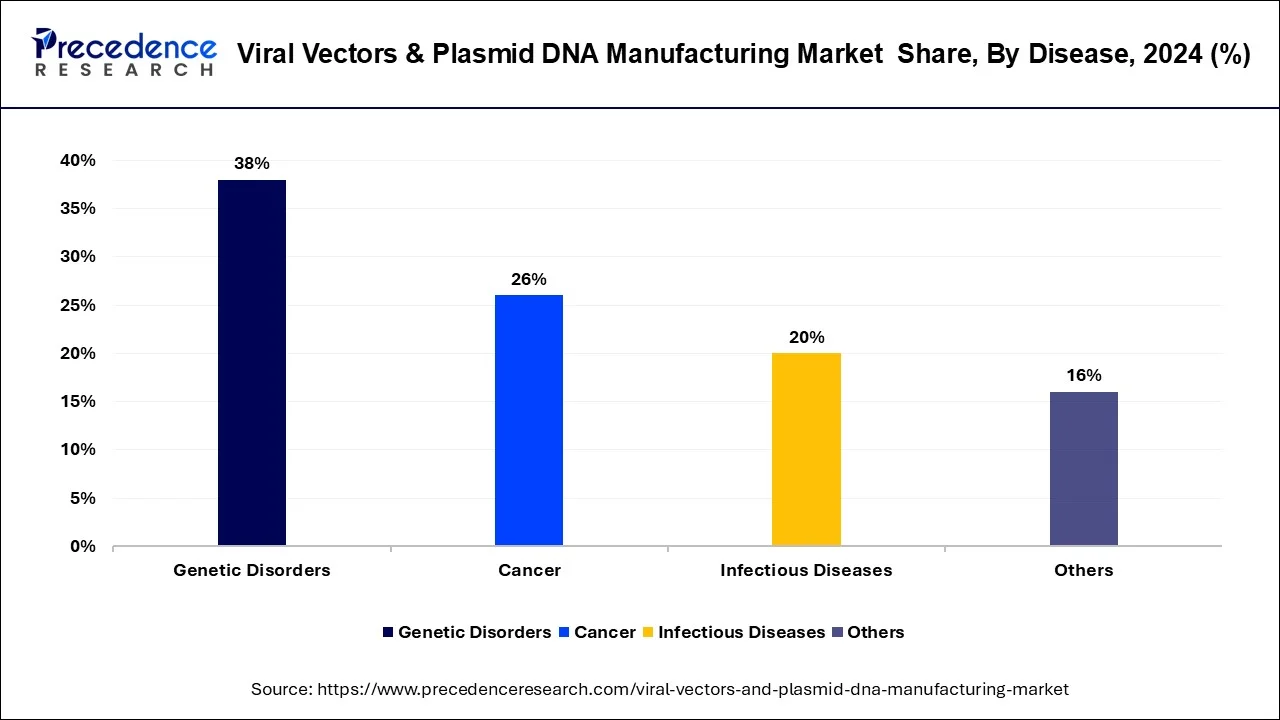
Cancer is projected to gather maximum share of the global market on account of continuously rising research on viral vector gene therapies for cancer. Furthermore, rare and genetic diseases are additional crucial area of attention in gene therapy. Due to almost 350 million patients are diagnosed worldwide with a rare disease, and there are deficiency of efficient treatment modalities. Constantly rising burden of genetic diseases is a crucial driving factor for venture in viral vector manufacturing in order to target genetic disease.
This research report estimates revenue growth at global, regional, and country levels and offers an analysis of present industry trends in everysub-segment from 2025 to 2034. This research study analyzes market thoroughly by classifying global viral vectors & plasmid DNA manufacturing market report on the basis of different parameters including type of vector, application, workflow, end users, disease, and region:
By Vector Type
By Application
By Workflow
By End-User
By Disease
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
October 2024
February 2025
January 2025
February 2025