January 2025
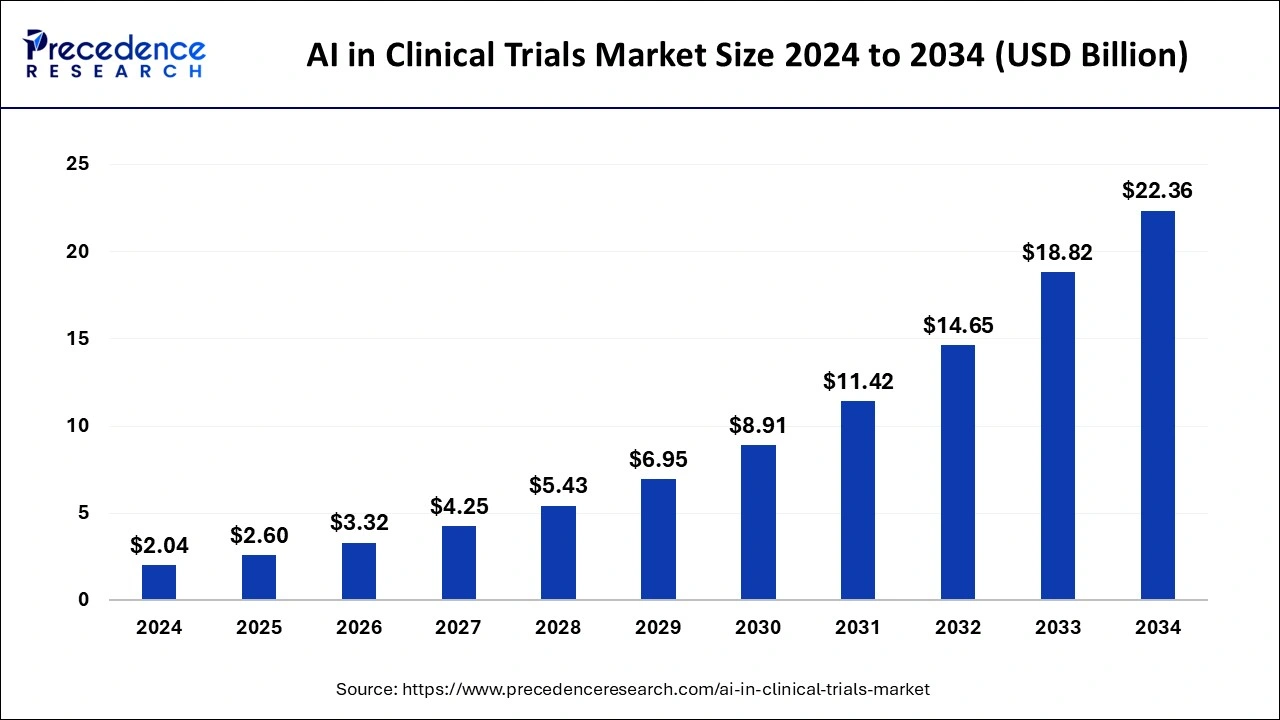
The global AI in clinical trials market size is calculated at USD 2.60 billion in 2025 and is forecasted to reach around USD 22.36 billion by 2034, accelerating at a CAGR of 27.05% from 2025 to 2034. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global AI in clinical trials market was estimated at USD 2.04 billion in 2024 and is predicted to increase from USD 2.60 billion in 2025 to approximately USD 22.36 billion by 2034, expanding at a CAGR of 27.05% from 2025 to 2034. The AI in clinical trials market is driven by the urgent requirement to speed up medication development and cut expenses.
Artificial Intelligence (AI) in clinical trials can revolutionize the entire drug development process. AI algorithms can quickly and cheaply sort through enormous amounts of data to find individuals who meet the requirements, cutting down on recruitment expenses and time. AI streamlines data analysis, boosting insights from large datasets and revealing hidden patterns. Artificial intelligence (AI) can evaluate past data and forecast future results, helping researchers create more successful and productive studies. AI can customize a patient's course of care according to their genetic and health characteristics. AI algorithms can swiftly identify adverse occurrences and improve patient safety by continuously analyzing medical data.
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 27.05% |
| Global Market Size in 2025 | USD 2.60 Billion |
| Global Market Size by 2034 | USD 22.36 Billion |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | By Offering, By Technology, By Application, and By End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Growing trend towards personalized medicine
Artificial intelligence (AI) is essential to personalized medicine because it can analyze large amounts of patient data, spot trends, and forecast each patient's unique reaction to a given medication. AI speeds up patient enrolment, simplifies data processing, and makes it easier to find patients who might be good candidates for tailored treatments in clinical trials. AI assists researchers in identifying biomarkers, stratifying patient populations, and optimizing trial design for more focused and effective results through machine learning algorithms.
AI systems can forecast how patients will react to therapies, which aids in the more effective and focused design of clinical trials. Drug development can go more quickly and economically by optimizing trial resources by recognizing potential responders and non-responders early in the process.
Artificial intelligence (AI) is used in clinical trials to speed up medication development and improve treatment precision and efficacy by customizing the regimen to each patient's needs. Clinical trial advances are fueled by the synergy between AI and personalized medicine, resulting in more individualized and potent therapeutic interventions.
Increasing recognition of AI in clinical trials by regulatory agencies
Regulatory bodies, like the food and drug administration (FDA) and other international equivalents, are becoming more aware of how artificial intelligence (AI) might improve and accelerate certain sections of clinical studies.
This recognition results from AI technologies' capacity to simplify and enhance clinical trial procedures, improving productivity, financial viability, and data accuracy. Artificial intelligence (AI) applications in clinical trials can significantly improve patient recruitment, data analysis, predictive modeling, and adverse event monitoring.
Standardization of AI models
The development of customized AI models is necessary because of the diversity of healthcare data, which includes patient information, imaging data, and electronic health records. However, developing broadly applicable AI models in the context of clinical trials is a challenge due to the requirement for standardized protocols and frameworks.
Utilizing disparate data formats, gathering techniques, and standards by healthcare establishments can impede the smooth incorporation of artificial intelligence solutions in various contexts. Regional ethical and regulatory limitations further compound the problem of standardizing AI models for clinical trials.
Hyper-personalized medicine and trial design
With AI, highly customized treatment regimens may be created by analyzing massive amounts of patient data, including genetic information, lifestyle factors, and medical history. This method improves treatment efficacy by considering each person's unique response to therapy.
AI makes identifying patient groupings based on traits easier, enabling more accurate and focused clinical trial recruitment. This shortens trial schedules and increases the chance of finding significant treatment benefits in patient groups. AI-enabled hyper-personalized medicine improves patient outcomes and satisfaction. This can decrease clinical trial dropout rates, which would lessen the requirement to find new patients and increase the validity of study findings.
Democratizing clinical trial participation
By pairing qualified individuals with large datasets, artificial intelligence (AI) can assist in finding and recruiting a wider variety of participants. Doing this ensures that clinical trials represent the variety of patients in the actual world. Artificial intelligence (AI) makes it easier to incorporate real-world data into clinical trials, giving researchers a more thorough grasp of how well a treatment works in various demographics and real-world situations. AI systems can quickly examine electronic health records and other healthcare data to find possible participants. This shortens trial times and related expenses while quickening the hiring process.
The services segment held the largest share of the AI in clinical trials market in 2024. Services provide customized solutions that fit into the workflows of current clinical trials. By customizing, AI applications are made to meet the unique requirements and goals of various clinical trials and healthcare institutions. When it comes to negotiating the regulatory environment around clinical trials, service providers are essential. They guarantee that AI applications abide by pertinent healthcare laws and business norms, giving stakeholders peace of mind and reducing risk.
The deep learning segment held the largest share of the AI in clinical trials market in 2024. Massive amounts of complex and diverse data, including genetic data, electronic health records (EHRs), medical imaging, and other data about clinical trials, are excellent targets for deep learning processing and analysis capabilities.
More precise and effective analysis is made possible by deep learning models, especially neural networks, and the ability to extract pertinent characteristics and patterns from unprocessed data automatically. Deep learning algorithms make adaptive trial designs possible, which support real-time clinical trial data monitoring. In response to gathered data, clinical studies can be more effective and responsive by using adaptive trials to modify essential parameters, such as patient enrollment criteria or treatment regimens.
The infectious disease segment dominated the AI in clinical trials market in 2024, the segment is observed to continue the expansion during the forecast period. Complex data sets, such as genetic information, epidemiological data, and clinical trial outcomes, are frequently involved in infectious diseases. Large amounts of heterogeneous data may be processed and analyzed by AI effectively, enabling researchers to obtain new insights and make defensible conclusions. Artificial intelligence (AI) technology makes it possible to monitor infectious diseases in real-time, which helps in outbreak identification and response planning. This capacity is essential for successful clinical studies amid pandemics or epidemics.
The pharmaceutical segment dominated the AI in clinical trials market in 2024 and the segment is expected to sustain the dominance throughout the forecast period. Pharmaceutical corporations use AI algorithms to examine large amounts of clinical data quickly. This expedites the medication development process by helping to recognize patterns, forecast patient reactions, and optimize trial methods. Artificial intelligence is employed by the pharmaceutical business to detect and alleviate possible hazards linked to clinical studies. Predictive analytics supports proactive risk management and patient safety by estimating the probability of unfavorable outcomes.
North America held the largest share of AI in clinical trials market in 2024. North America, especially the United States, has led in the achievements of artificial intelligence innovation and technology. Numerous top IT firms, academic organizations, and startups that concentrate on creating innovative AI solutions for various industries, including clinical trials and healthcare, can be found in the region. It has made significant financial and investment commitments to AI businesses and research projects. Private investors, government funding, and venture capital firms have demonstrated a strong desire to assist the advancement and application of AI technology in healthcare, including clinical trials.
Asia-Pacific is expected to witness the fastest rate of expansion in the AI in clinical trials market during the forecast period. The market is expanding due to a growing patient base, a variety of patient demographics, and comparatively lower expenses than in Western nations. As a result, there is a greater need than ever for cutting-edge technologies, such as artificial intelligence (AI), for managing and carrying out clinical studies. Asia-Pacific's large and diversified patient populations offer a wealth of data for clinical trials. Artificial intelligence (AI) technologies can enhance the efficiency of data analysis, particularly in patient recruitment, stratification, and trial lifecycle monitoring.
By Offering
By Technology
By Application
By End-user
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
January 2025
November 2024
October 2024