March 2025
The global clinical trial investigative site network market size is calculated at USD 9.36 billion in 2025 and is forecasted to reach around USD 15.77 billion by 2034, accelerating at a CAGR of 5.97% from 2025 to 2034. The North America market size surpassed USD 4.50 billion in 2024 and is expanding at a CAGR of 5.98% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global clinical trial investigative site network market size accounted for USD 8.83 billion in 2024 and is predicted to increase from USD 9.36 billion in 2025 to approximately USD 15.77 billion by 2034, expanding at a CAGR of 5.97% from 2025 to 2034.
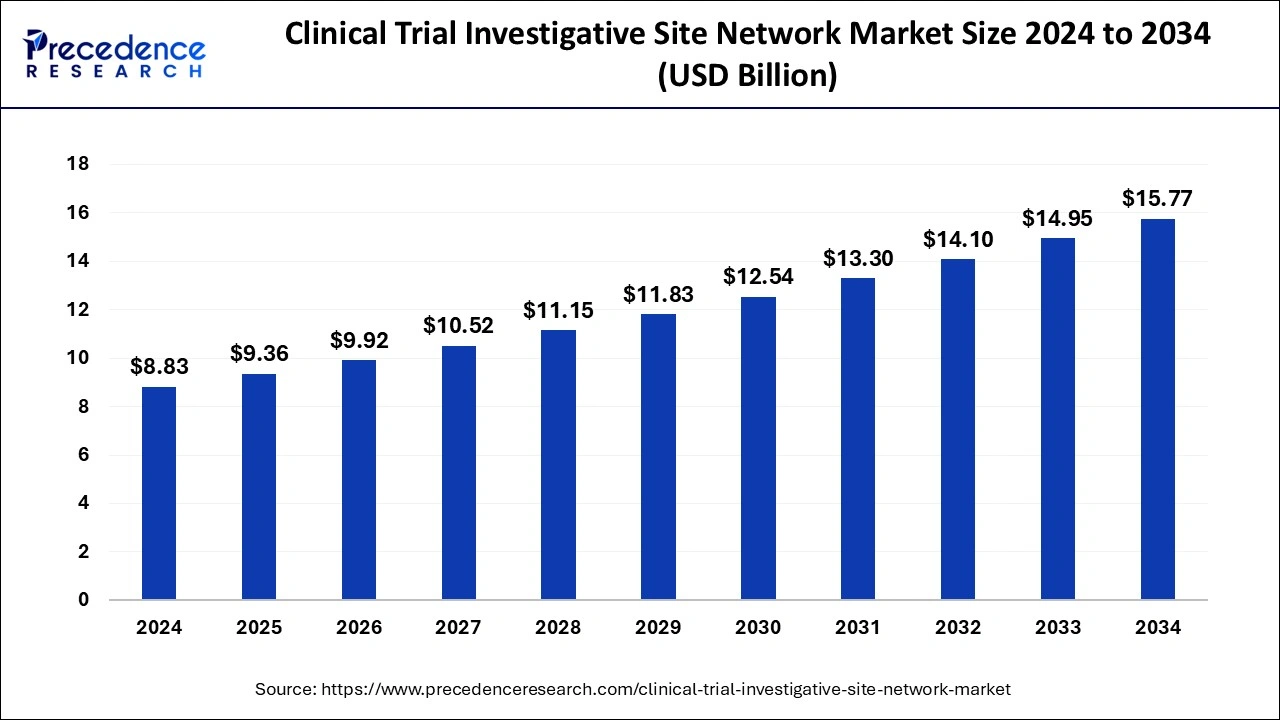
The U.S. clinical trial investigative site network market size was exhibited at USD 3.15 billion in 2024 and is projected to be worth around USD 5.68 billion by 2034, growing at a CAGR of 6.07% from 2025 to 2034.
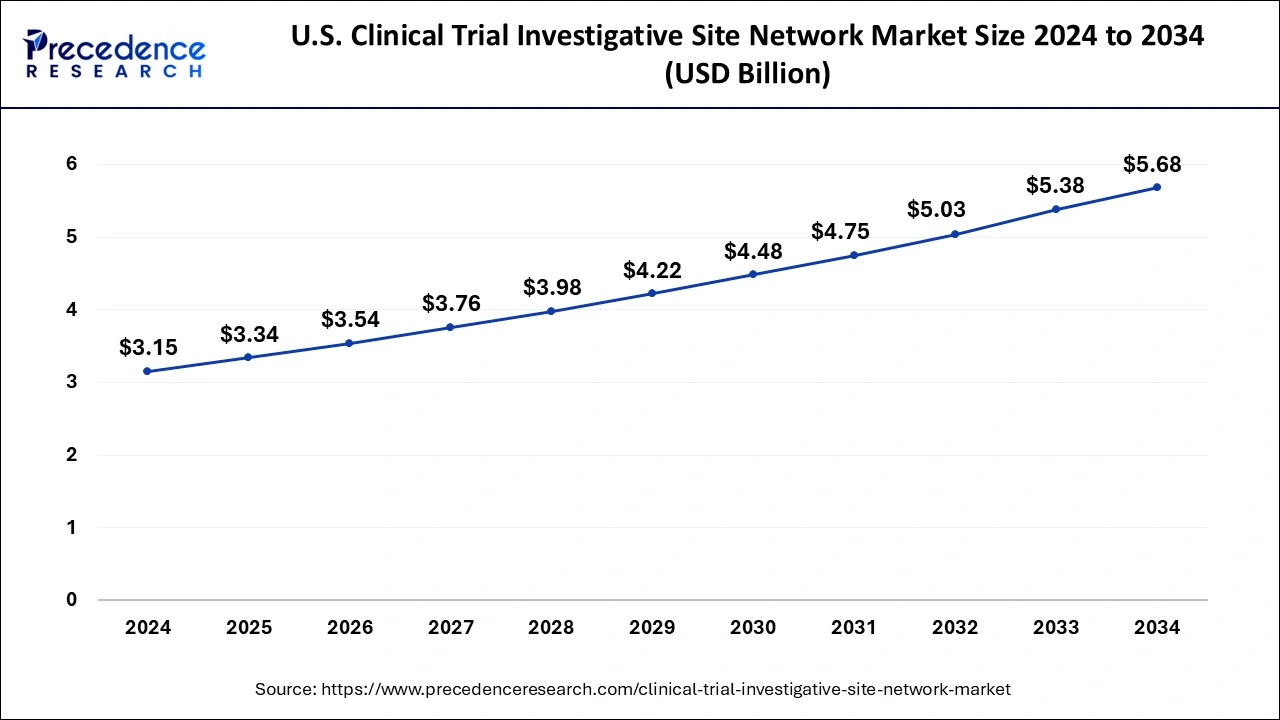
North America held the largest share of the clinical trial investigative site network market in 2024. The region is expected to grow significantly during the forecast period. This is due to various factors, such as a well-established healthcare infrastructure, technological advancements, a rise in clinical trials, and an increase in chronic diseases. These factors are contributing to the market's revenue in the region.
The U.S. clinical trial investigative site network market has witnessed a robust growth rate owing to the increasing number of companies offering owing to the rising interest in clinical trials, rapid advancements in medical technology, increasing patient population, increasing focus on rare disease treatment, and growing demand for new therapies. Additionally, the rising spending on clinical research by U.S. pharmaceutical companies, supportive government policies, and rising government funding for clinical trials are expected to fuel the expansion of the market in the region.
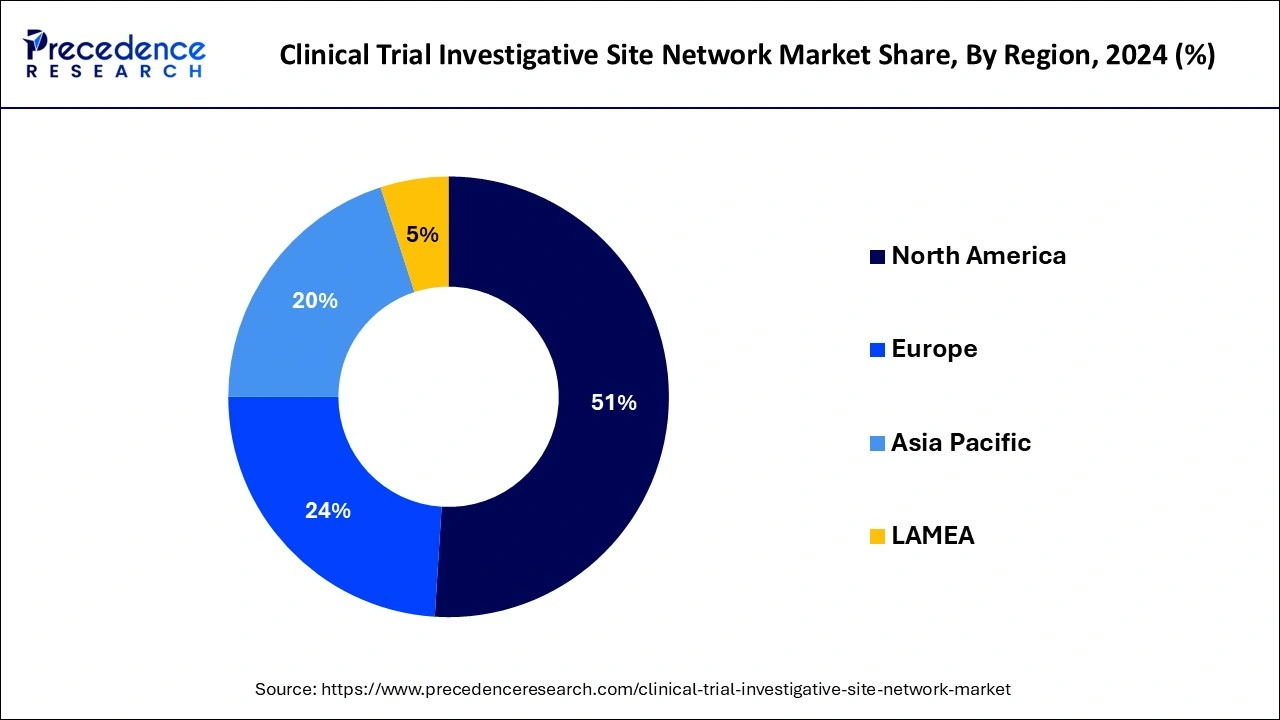
Asia Pacific clinical trial investigative site network market is observed to expand at a rapid pace during the forecast period due to several factors, including increasing investment in the healthcare infrastructure, increasing investments in R&D activities, and rising demand for new therapies for treating chronic illness. Moreover, the region has ease of regulatory compliance, fewer costs required in studies, a rising patient population with chronic illness, and the presence of renowned clinical institutions functioning as sites. Furthermore, the governments actively participate in improving R&D activities by providing tax deductions.
Clinical trials are conducted in several phases to answer specific questions and ensure the safety of participants. These trials are carried out in accordance with the Good Clinical Practice (GCP) guidelines mandated by regulators to protect patients. Generally, a new treatment is tested in three to four phases of clinical trials before regulatory agencies consider it safe and effective. Clinical trials provide valuable information on the potential treatment, its associated risks, and various aspects of the patient's quality of life.
The clinical trial investigative site network market encompasses collaboration between contract research organizations (CROs) and sponsors for conducting clinical trials. Clinical investigational site networks are a group of independent clinical sites that meet certain qualifying criteria and work as a single entity, saving R&D expenses. Research centers, clinics, hospitals, and other specialized facilities that fulfill the precise trial standards can be used as sites. These sites are independently owned and operated, which distinguishes investigative site networks from other outsourcing businesses, such as contract research organizations and site management firms.
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 5.97% |
| Market Size in 2025 | USD 9.36 Billion |
| Market Size in 2024 | USD 8.83 Billion |
| Market Size by 2034 | USD 15.77 Billion |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Therapeutic Areas, Phase, and End-use |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Increase in the number of clinical trials
The rise in the number of clinical trials is a major driving factor accelerating the growth of the clinical trial investigative site network market. Clinical trials assist in advancing patient treatment and medical care. Clinical trials are also used to test new diagnostic techniques for diseases, often even before their first symptoms start to harm the body. Clinical trials may also be conducted to test methods of disease prevention. Clinical trials may also evaluate the efficacy of strategies intended to help those with chronic illnesses or life-threatening diseases feel better physically.
Clinical trials are typically governed by ethics committees and health authorities such as the European Medicines Agency (EMA), the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, the U.S. Food and Drug Administration (FDA), and others. Hiring a clinical trial investigative site network empowers the regulatory function and improves the enrollment of participants, data management, and quality assurance. It enhances process compliance, reduces issues associated with each trial, and assists with faster trial initiations and shorter trial periods. Therefore, the increasing incidence of chronic diseases around the globe has resulted in a rise in several clinical trials, propelling the growth of contract research organizations.
High cost
The high cost associated with clinical trials is anticipated to hamper the market's growth. Compliance with stringent regulatory regulations is expensive and time-consuming for investigative site networks. During clinical trials, patient recruitment and retention are not always easy. In addition, the strict guidelines regarding eligibility requirements, consent of patients, and trial competitiveness are some of the factors that may restrict the expansion of the global clinical trial investigative site network market.
Rapid advancement in medical technology
The rapid advancement in technology is projected to offer a lucrative opportunity for the growth of the global clinical trial investigative site network market during the forecast period. The robust growth in medical technology for advancing chronic illness treatments is gaining momentum globally. Investigational site networks are in demand owing to the need to conduct clinical studies to assess the efficacy of these innovative technologies. Health professionals and authorized government bodies are to recognize the significance of evidence-based medicine. Thus, for any novel medications to be approved for use, they must contain proof from clinical trials, which leads to increasing demand for investigative site networks.
The oncology segment accounted for the dominating share of the clinical trial investigative site network market in 2024 and is also projected to continue its dominance over the forecast period. This is due to the rising research on novel cancer drug therapies. The growth of the segment is majorly driven by the increasing prevalence of cancer disease, which escalates the demand for cancer trials in research.
The pain management segment is expected to witness considerable growth in the clinical trial investigative site network market over the forecast period. The growing cases of chronic pain are the major factors expected to drive the growth of the segment. With the growing emergence of new disorders, there is a growing requirement for therapeutic solutions.
The phase III segment held the largest share of the clinical trial investigative site network market in 2024 and is expected to sustain the position throughout the forecast period. Clinical studies conducted in phase III are more complicated than in earlier stages. Phase III includes a larger pool of patients than other phases, and this phase also includes the highest failure rate due to the sample size and research design necessitating precise dosing at an optimal level. Before any novel medicine is approved, studies are frequently undertaken in bigger populations in several locations and nations. Such factors accelerate the growth of the segment.
The phase I segment is expected to grow significantly during the forecast period. These studies examine the tolerability and pharmacokinetics of molecules as they analyze a product's safety. It establishes how a device or medication will affect people, including how it will be absorbed, metabolized, and eliminated. Thus, fueling the segment’s growth.
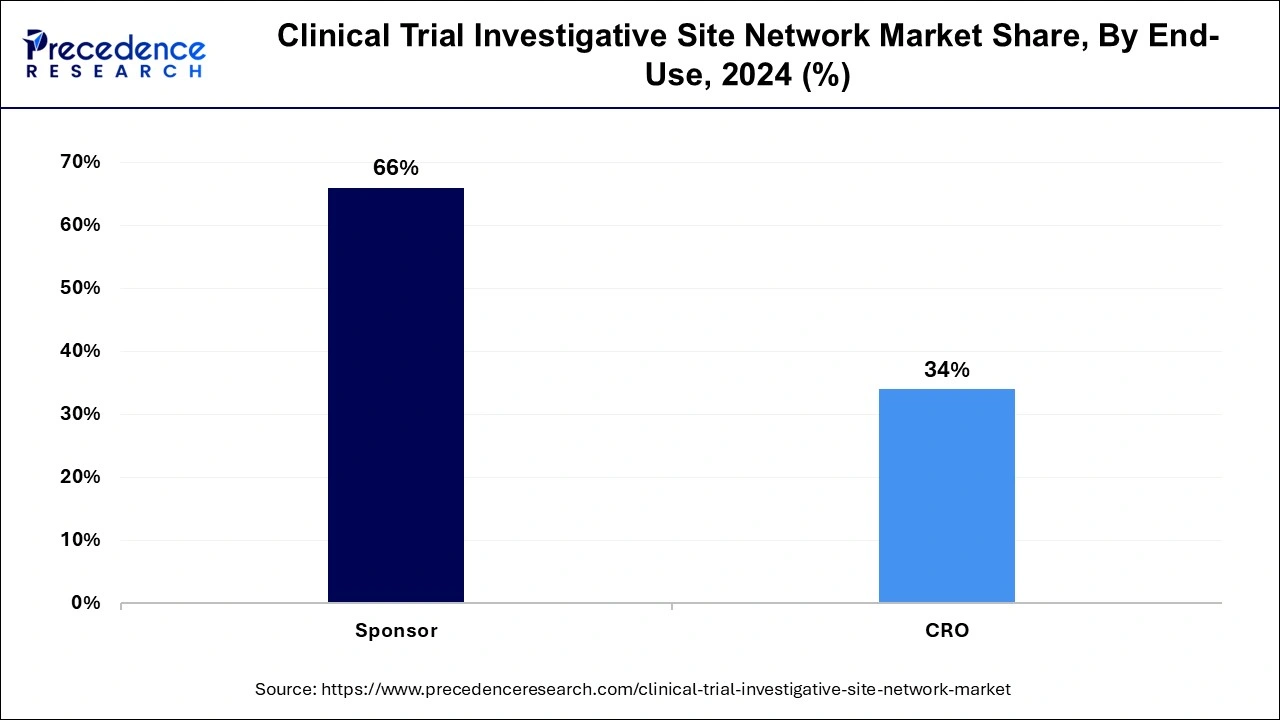
The CRO segment held the largest share of the clinical trial investigative site network market in 2024. segment is growing due to the increasing number of key sponsors, including pharmaceutical companies, biopharmaceutical companies, and medical device companies. The market has witnessed a high amount of funding for clinical research by the sponsors. On the other hand, the CRO segment is expected to grow fastest during the forecast period owing to the increasing number of contract research organizations offering clinical trial management services.
By Therapeutic Areas
By Phase
By End-use
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
March 2025
February 2025
February 2025
January 2025