February 2025
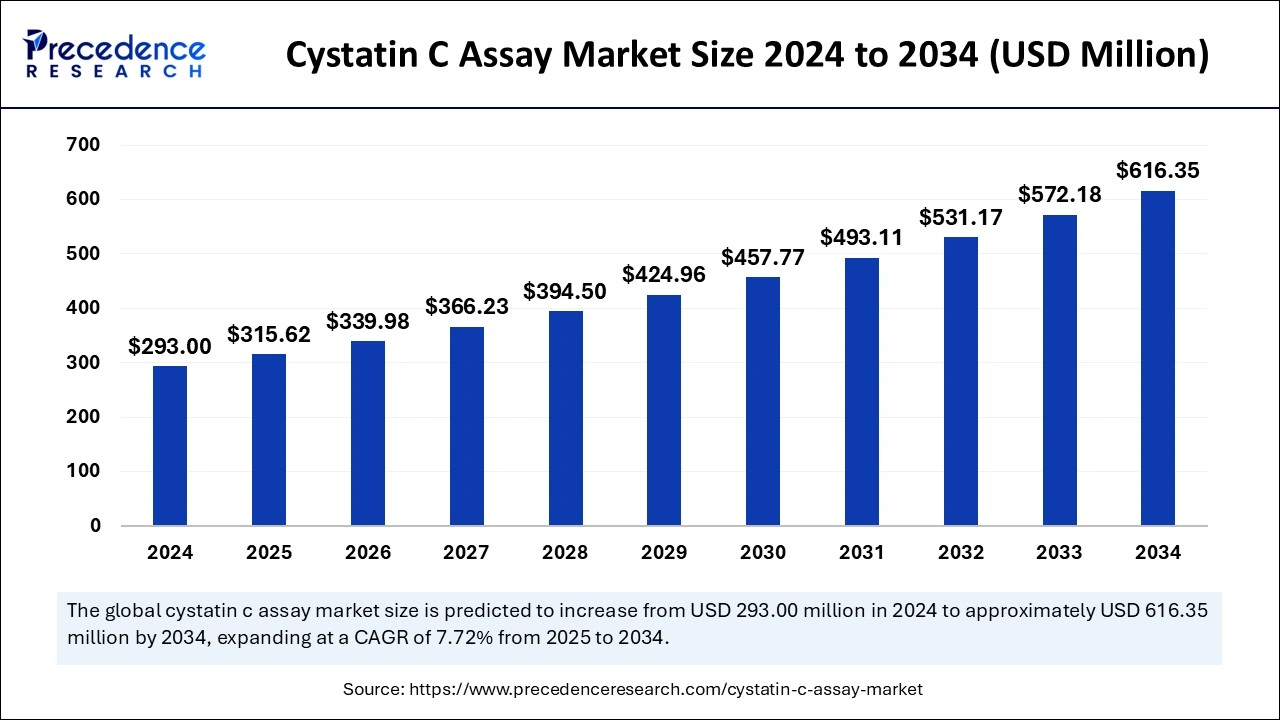
The global Cystatin C assay market size is accounted at USD 315.62 million in 2025 and is forecasted to hit around USD 616.35 million by 2034, representing a CAGR of 7.72% from 2025 to 2034. The North America market size was estimated at USD 128.92 million in 2024 and is expanding at a CAGR of 7.84% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global Cystatin C assay market size was calculated at USD 293.00 million in 2024 and is predicted to reach around USD 616.35 billion by 2034, expanding at a CAGR of 7.72% from 2025 to 2034. The Cystatin C testing market is propelled by the growing prevalence of CKD, improvements in diagnostic technologies, enhanced test accuracy, and greater investments in healthcare.
Artificial intelligence and automated systems now help the Cystatin C assay market make better and faster diagnoses supported by predictive analysis. Technology lets AI systems link to medical records and lab systems to track kidney function results in real time. Personalized medicine uses AI information to better match Cystatin C tests to each patient's unique medical needs. AI technology improves Cystatin C test capabilities and drives new development while delivering better patient results and shaping kidney disease diagnosis.
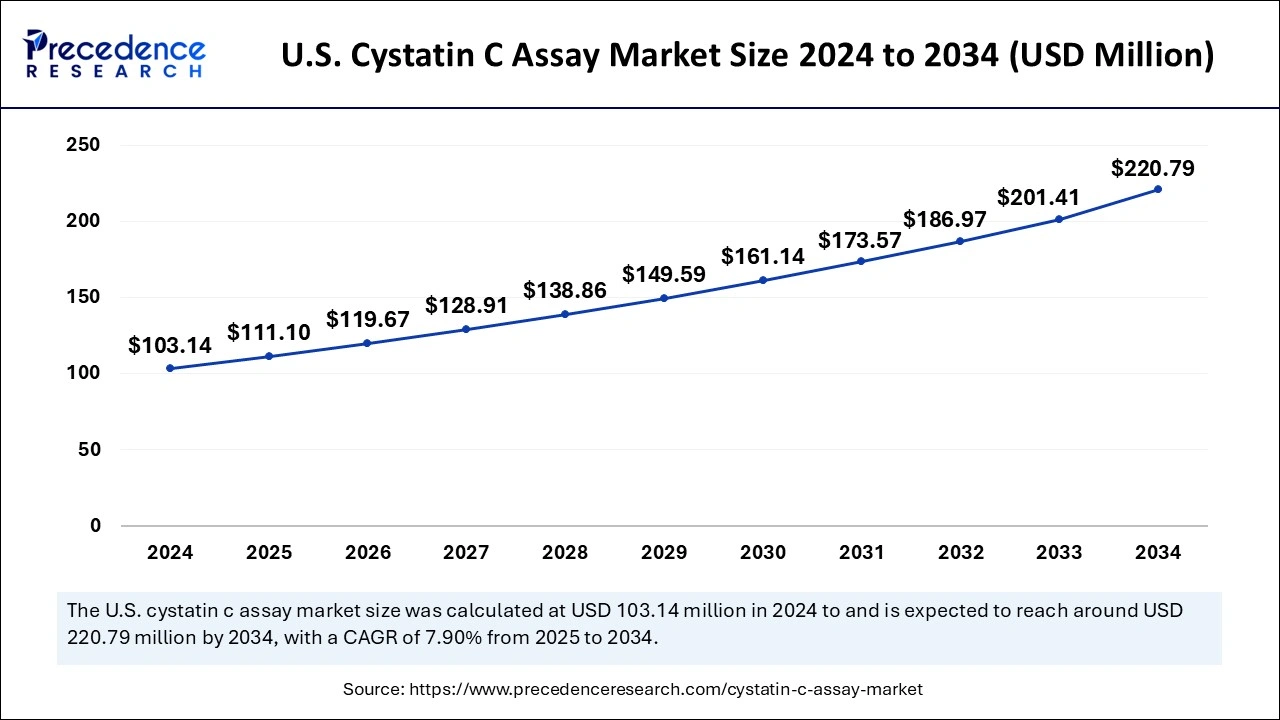
The U.S. Cystatin C assay market size was exhibited at USD 103.14 million in 2024 and is projected to be worth around USD 220.79 million by 2034, growing at a CAGR of 7.90% from 2025 to 2034.
North America accounted for the largest Cystatin C assay market share in 2024. Improved healthcare systems and fast adoption of new testing technologies make it easy to use Cystatin C tests. The strong presence of major players in the market, the extraordinary healthcare infrastructure, and the large pool of people having specifically kidney disorders. Chronic kidney diseases in North America are causing high demand for Cystatin C tests because they affect so many people with kidney problems.
Organizations and institutions continue to research new ways to improve blood tests for Cystatin C and make them work better in the North American market. North America actively promotes public knowledge programs about kidney health and teaches healthcare providers how to detect diseases at their early stages.
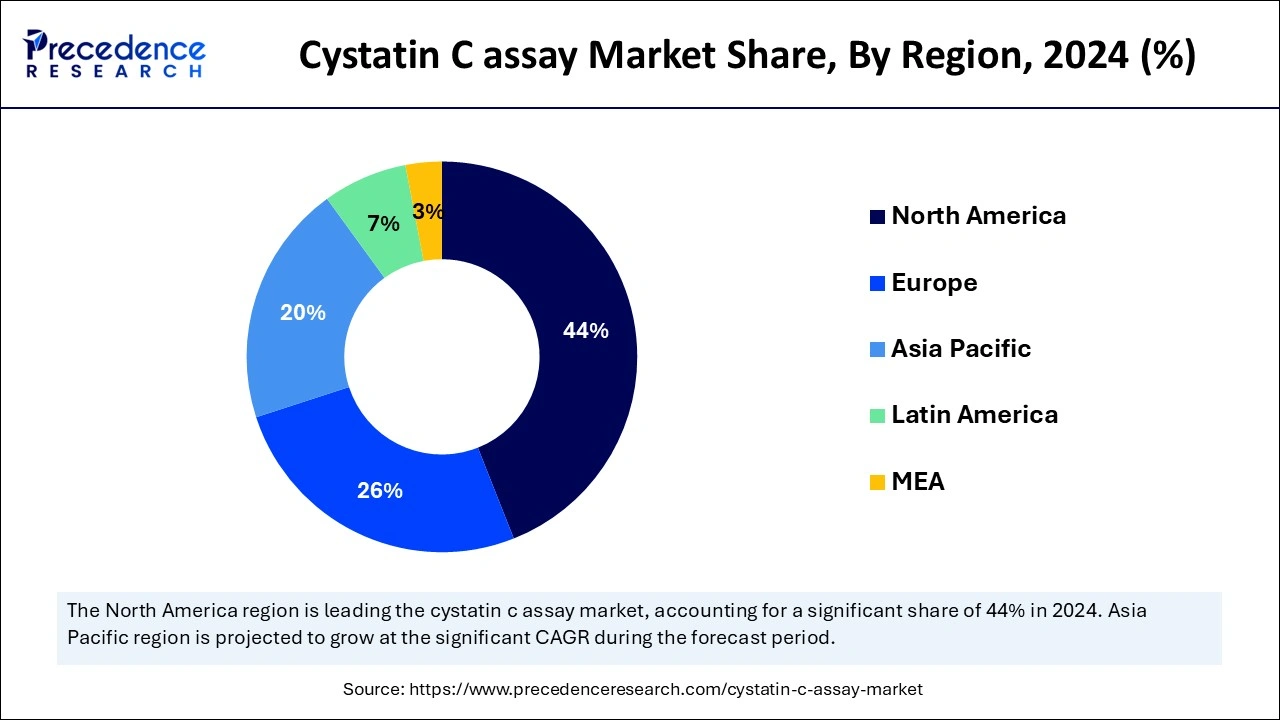
Asia Pacific is anticipated to witness the fastest Cystatin C assay market growth during the forecasted years. The region is experiencing rapid expansion due to rising healthcare spending that facilitates improved access to advanced diagnostic technologies. Moreover, governmental programs and regulations focused on enhancing healthcare quality and early disease identification support market acceptance.
The growth in disposable income, heightened health awareness, and the need for diagnostic tests, especially among the elderly, are expected to propel market growth in the area. CKD poses a significant health issue in countries like India and China. An increasing awareness among healthcare professionals and patients regarding the significance of early CKD detection aids the development of accurate kidney function assessment techniques.
A Cystatin C assay is a diagnostic test that assesses the level of Cystatin C, a protein generated by all nucleated cells in the blood. Cystatin C works as a reliable sign of kidney health in measuring glomerular filtration rate (GFR). Cystatin C measurements show lower dependency on age, gender, and muscle mass factors, which makes this test a better indicator of kidney health status. When Cystatin C increases in blood, it shows that the kidneys are less effective, which raises the chances of heart problems and death. Doctors use this test regularly to follow how kidney conditions develop.
The higher CKD rates from aging populations and increased diabetes and hypertension numbers push industry demand forward for Cystatin C tests, which measure kidney function well. The development of advanced Cystatin C assay technology with automatic systems and sensitive tests has driven market growth. The Cystatin C assay market continues to grow due to improved regulations for testing standards and investment in healthcare systems across developing nations.
| Report Coverage | Details |
| Market Size by 2024 | USD 293.00 Million |
| Market Size in 2025 | USD 315.62 Million |
| Market Size in 2034 | USD 616.35 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 7.72% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product, Sample Type, Method, Application, End-User, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Increasing occurrence of kidney disorders
Chronic kidney disease rates increase along with the aging population, which pushes the Cystatin C assay market forward. Poor kidney function affects the body by causing anemia while weakening immune defenses and lowering appetite plus calcium levels. Additionally, the elevated risk of CKD in patients with diabetes, hypertension, and heart conditions, coupled with the rising incidence and death rate of these illnesses, will favorably influence market expansion. The rising prevalence of kidney diseases highlights the need for accurate and reliable diagnostic methods, like Cystatin C assays, for the early detection, monitoring, and effective treatment of renal conditions.
Cost concerns
The global Cystatin C assay market, which provides advanced diagnostic tools for evaluating kidney function and early identification of kidney diseases, encounters a major challenge due to cost issues. However, Cystatin C tests offer enhanced accuracy and sensitivity. They have comparatively higher costs. The expensive nature of Cystatin C tests prevents their frequent use in healthcare systems with funding constraints or spending controls. Healthcare facilities must evaluate how Cystatin C tests affect their financial plan, especially when working with multiple patients or when alternate diagnostic options exist.
Increased adoption of point-of-care testing (POCT)
The development and use of Cystatin C tests for point-of-care tests improve kidney function monitoring access in primary care settings and emergency rooms, which will provide additional benefits for rural patients who need care. Point-of-care tests supply an important testing option by making rapid portable Cystatin C monitoring available in medical locations besides labs. Providing rapid outcomes, these tests allow for instant clinical decisions at the patient’s bedside or in distant areas. POCTs are especially advantageous in emergency departments, outpatient care facilities, and primary care offices, facilitating prompt evaluation of kidney function for efficient patient management.
The kits segment accounted for the largest share of the Cystatin C assay market in 2024. These kits supply complete testing tools to both laboratories and healthcare personnel for conducting Cystatin C measurements. Easy kit use without extensive training makes Cystatin C tests available across different healthcare settings. Different medical facilities can use Cystatin C Kits either for low-volume manual tests or for high-volume automated procedures. Healthcare professionals can choose the right Cystatin C kit for their environment, making this test accessible to many different medical facilities. Standardized Cystatin C test kits help labs throughout the healthcare system produce similar results for better patient diagnoses and treatment.
The reagents segment is projected to grow at the fastest CAGR during the forecast period. The Cystatin C assay market refers to the sector dealing with reagents and assays that measure the levels of Cystatin C in biological specimens. Clinical laboratories and research organizations require specific reagents because their work demands unique requirements. Specific testing materials serve essential purposes in both medical laboratories and scientific research facilities. High-grade testing reagents help deliver accurate results in Cystatin C tests as the market develops.
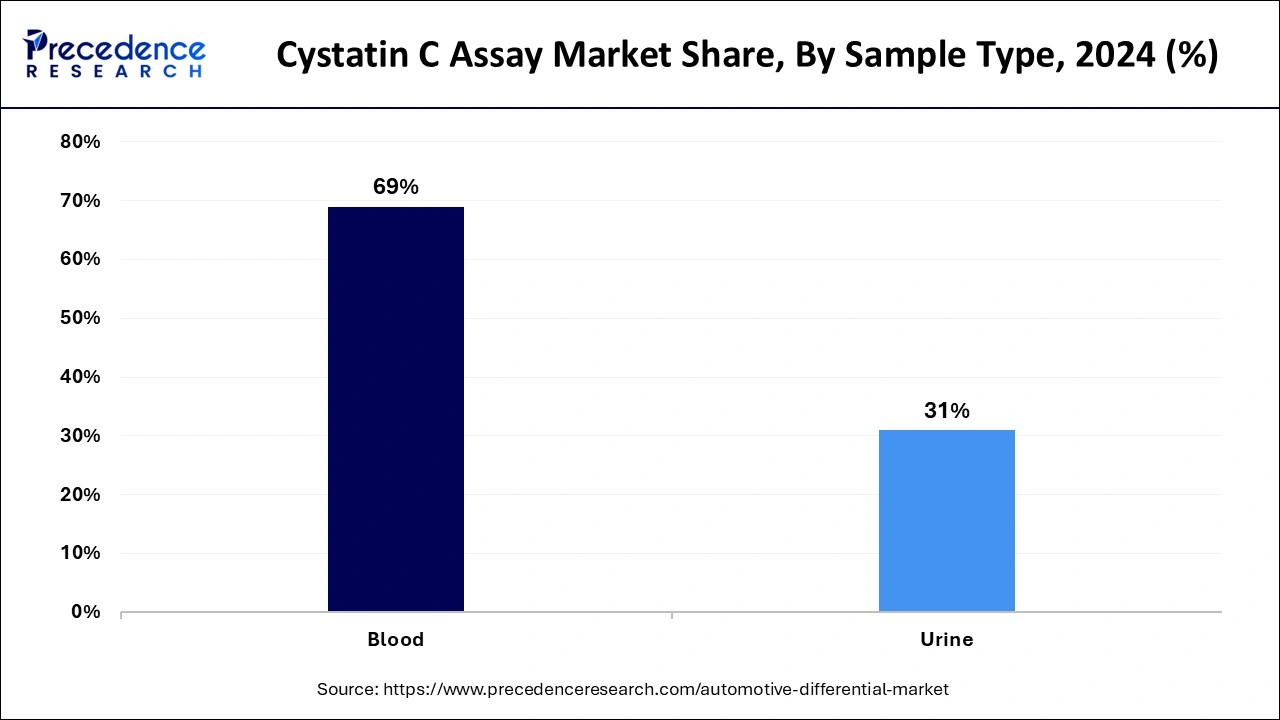
The blood segment noted the largest Cystatin C assay market share in 2024. Scientific labs and hospitals rely heavily on blood samples for testing and research. The increase in chronic kidney disease and acute kidney injury cases across society makes blood-based Cystatin C tests essential for better medical results.
The Cystatin C blood test assists physicians in identifying kidney issues early on to initiate effective treatment and enhance patient results. Healthcare professionals opt for blood-based Cystatin C tests to identify kidney issues sooner as they recognize the importance of ongoing assessment of organ function.
The enzyme-linked immunosorbent assay dominated the global Cystatin C assay market in 2024. ELISA stands as a highly reliable and broadly accepted technique in immunoassay science. The technique shows strong diagnostic value in studies and healthcare settings, making it trusted across labs and care services. The enzyme-linked immunosorbent assay shows high measurement accuracy to find small amounts of Cystatin C in medical samples. The ELISA process detects different forms of Cystatin C, including Cystatin C2 through its flexible design. Healthcare experts and researchers depend on this testing system because it works well for different medical needs.
The diagnostics segment led the global Cystatin C assay market in 2024. Healthcare providers use Cystatin C tests to detect early signs of kidney problems. Medical facilities and research teams require reliable testing methods for CKD and AKI patients because these diseases are growing in numbers worldwide. The rising focus on personalized medicine has heightened the need for precise biomarkers like Cystatin C, which can offer valuable information about individual patient profiles, aiding in tailored treatment approaches and enhancing patient results. Increasing global healthcare spending, along with investments in research infrastructure, encourages the adoption of advanced diagnostic technologies and partnerships between diagnostic companies and research organizations.
The hospital segment held the largest Cystatin C assay market share in 2024. More patients benefit from testing because medical facilities are easy to reach. Hospitals perform Cystatin C tests under defined quality management processes. Medical facilities rely on Cystatin C testing to detect diseases and track treatment progress, while diagnostic laboratories provide advanced testing for specific patient conditions. These factors drive the highest segment growth because they treat many patients and can easily visit the facilities.

By Product
By Sample Type
By Method
By Application
By End-Use
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
February 2025
March 2025
August 2024
March 2025