January 2025
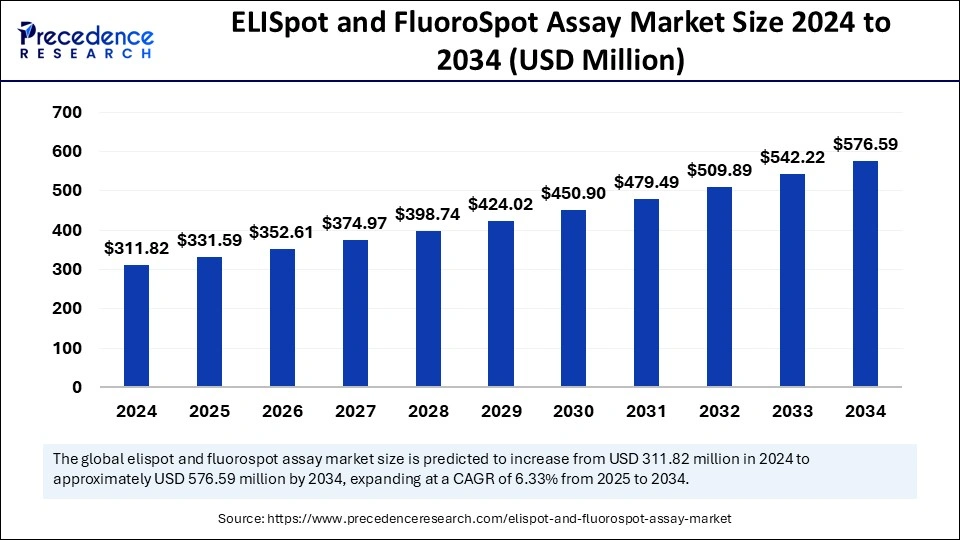
The global ELISpot and FluoroSpot assay market size is calculated at USD 331.59 million in 2025 and is forecasted to reach around USD 576.59 million by 2034, accelerating at a CAGR of 6.33% from 2025 to 2034. The North America market size surpassed USD 109.14 million in 2024 and is expanding at a CAGR of 6.49% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global ELISpot and FluoroSpot assay market size accounted for USD 311.82 million in 2024 and is predicted to increase from USD 331.59 million in 2025 to approximately USD 576.59 million by 2034, expanding at a CAGR of 6.33% from 2025 to 2034. The growth of the ELISpot and FluoroSpot assay market can be linked to the growing demand for advanced diagnostics solutions, increased focus on research activities for understanding disease mechanisms and increased adoption of advanced assay technologies.
Implementation of artificial intelligence in ELISpot and FluoroSpot assays is increasing accuracy and reproducibility, improving efficiency and enhancing the data analysis for quantifying complex immune responses. AI algorithms can be applied for automating the counting and analysing the spots in assays, for analyzing morphologies of spots such as intensity, location, size and circularity for identification of single, double and triple-secreting cells as well as in multi-color analysis of complex data generated of FluoroSpot assays. AI-driven tools such as IntelliCount which is an AI-powered counting algorithm applied for observing antigen-specific B-cell assays and Exploraspot for the detection and quantification in automated fluorescence microscopic images for FluoroSpot assays.
Furthermore, machine learning algorithms can be applied in ELISpot assays for identification of cytokine-secreting cells, in diagnosing infectious diseases such as sarcoidosis and tuberculosis, for cancer immunotherapy research and in detection of antigen-specific T-cells. Also, in FluoroSpot assays, machine learning methodologies are implemented for quantification of analyte-secreting cells and in sensitive sandwich assay which helps in analyzing data for identifying patterns and specific trends in immune responses.
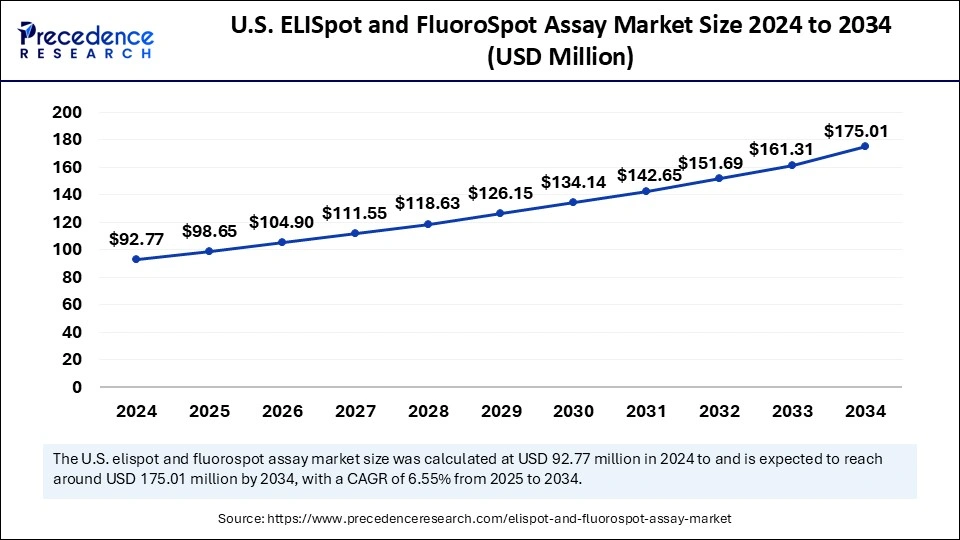
The U.S. ELISpot and FluoroSpot assay market size was exhibited at USD 92.77 million in 2024 and is projected to be worth around USD 175.01 million by 2034, growing at a CAGR of 6.55% from 2025 to 2034.
North dominated the global ELISpot and FluoroSpot assay market with the largest share in 2024. The market dominance of this region is driven by the presence of advanced healthcare infrastructure, robust research and development activities, stringent regulations, increased funding for biomedical research, huge number of ongoing clinical trials for oncology and other diseases, rising disposable incomes and surge in number of diagnostic tests for various infectious diseases and other disorders.
U.S. dominated the ELISpot and FluroSpot assay market in North America with the largest share in 2024. Major pharmaceutical and biotechnology companies heavily investing in vaccine research and drug discovery, supportive government initiatives, accelerated approvals from the Food and Drug Administration (FDA), ongoing advancements for developing innovative platforms and presence of research organizations and academic institutes are the factors driving the market growth. Additionally, according to the U.S. National Library of Medicine (NLM), about 20,465 patients were recruited for clinical trials till January 2024 in the U.S. which drives the demand for ELISpot and FluoroSpot assays.
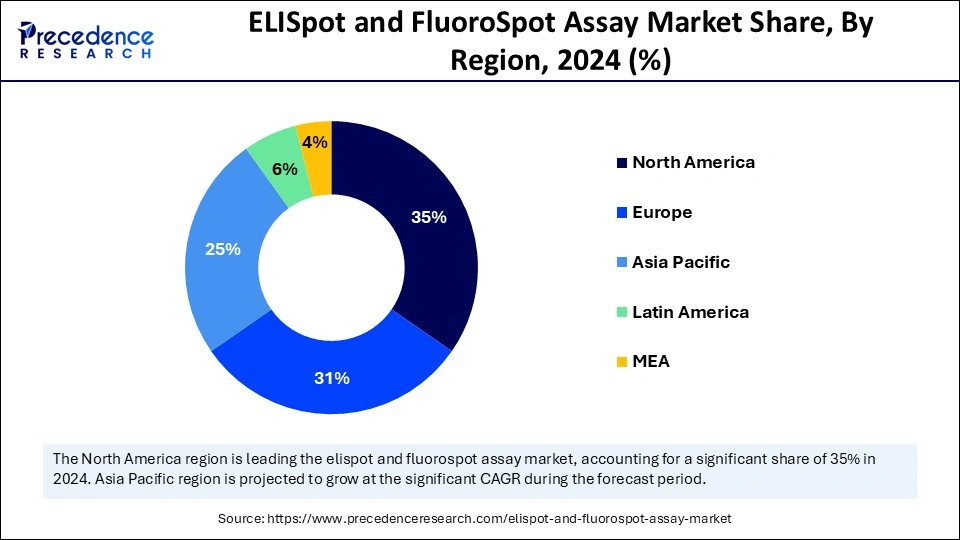
Asia Pacific region is anticipated to witness lucrative growth in the market over the forecast period. The increased expenditure for the development of advanced healthcare facilities and diagnostic laboratories, rising investments in immunological research for developing immunotherapies and vaccines, expansion of the biopharmaceutical industries, large number of ongoing clinical trials driven by the diverse demographics, funds sponsored by governments for biomedical research and surge in cases chronic and infectious diseases like hepatitis B, diabetes, tuberculosis and cancer are the factors promoting the market growth of this region.
China is expected to show the fastest growth in the market during the forecast period. The market growth is driven by the large population base, rising cases of infectious diseases, growing demand for advanced and practical diagnostic tools for developing personalized treatment strategies. Additionally, supportive government initiatives such as for expanding the biopharmaceutical sectors, funding for biomedical research and the Made in China 2025 initiative for transforming China into global manufacturing powerhouse are expanding the market.
Europe is predicted to witness notable growth in the ELISpot and FluoroSpot market in the upcoming years. The presence of strong research infrastructure, growing disease burden, rising investments for expanding R&D operations, supportive regulatory environment and increased focus of companies on geographical expansion such as for improving product portfolios with the acquisition of local manufacturers are the factors fostering the growth of this region.
The UK market for ELISpot and FluoroSpot assays is expected to grow significantly in the upcoming years. The market growth can be attributed to the rising investments in immunology research, increased awareness for early disease diagnosis in patients and availability of advanced diagnostic tools and the growing geriatric population.
The ELISpot (Enzyme-Linked Immunospot) assay is a variation of the enzyme-linked immunosorbent assay (ELISA) and is highly sensitive method utilized for the detection and quantification of immune cells producing specific cytokine or antibodies which can be visualized as spots created after a reaction with enzymes and substrates. The assay provides both qualitative data like which molecules are secreted as well as quantitative data like how many cells are secreting them. Furthermore, FluoroSpot assays are an extension of the ELISpot assay utilizing fluorescently labelled detection reagents for the synchronous detection of numerous secreted analytes in a single well.
These assays are widely used for various application such as in large-scale epitope mapping, for detection of HLA (Human Leukocyte Antigen)- restricted responses, for measuring immune responses of T-cells and B-cells from PBMC (Peripheral Blood Mononuclear Cells), for evaluating vaccine efficacy, in detection of rare antigen-specific T-cells among others.
The ongoing advancements in diagnostic technologies, increased research activities for drug discovery and immunotherapies, expansion of product portfolios by companies with the development of innovative platforms, automation of workflows, burgeoning demand for assay kits and analyzers are the factors driving the growth of the ELISpot and FluoroSpot assays market.
| Report Coverage | Details |
| Market Size by 2034 | USD 576.59 Million |
| Market Size in 2025 | USD 331.59 Million |
| Market Size in 2024 | USD 311.82 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 6.33% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product , Application, End Use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Ongoing advancements in diagnostic technologies
The growing geriatric population, rising demand for advanced diagnostic tools for accurate detection of chronic and infectious diseases, increased patient awareness for early disease diagnosis to improve patient outcomes and increased use of assays for diagnostic and research applications driving the market growth. Advancements in ELISpot and FluoroSpot assays such as upgraded kits with quality control measures, pre-coated plate development for ELISpot assay, multi-analyte detection in FluoroSpot assays and development of multiplexed FluoroSpot assays such as the FluoroSpot Plus platform developed by Mabtech allowing polyfunctional T-cell profiling, enhanced B-cell profiling and also captures and detects monoclonal antibodies (mAb) are all improving the sensitivity, standardization and reducing workloads of processes.
Furthermore, these assays are also applied for epitope mapping, for monitoring vaccine-induced T and B-cell responses, for identifying tumor antigens and in the detection and characterization of naturally occurring tumor-reactive T-cells for cancer immunotherapy. These factors contribute to the growth of the ELISpot and FluoroSpot assays market.
Lack of skilled personnel and high costs
Although the ongoing technological advancements in assay technologies have improved the accuracy and user convenience of these methods, they still require skilled expertise for the better understanding and interpretation of generated data to make timely and informed decisions without any errors further limiting their accessibility in some settings.
Moreover, the high costs associated with the adoption, maintenance of analyzers and for buying the assays kits and reagents can potentially deter the market growth, especially in constrained-resource environments. Also the availability of various other detecting technologies can hinder the market growth.
Growing applications creating lucrative opportunities
The ELISpot and FluoroSpot assays are extensively being applied for various applications owing to the factors such as increased incidences of chronic and infectious diseases, rising investments for expanding research and diagnostic applications, ongoing advancements in drug discovery and biotechnology industries and government support. These assays are applied for monitoring immune responses, for diagnosis of infectious diseases, in cancer immunotherapy research and for detection of cytokine-secreting cells among others. Furthermore, increased adoption of these techniques in emerging economies and growing focus of companies on developing assay kits and advanced analyzers are expected to create lucrative opportunities for growth of the market.
Assay kits segment dominated the market with the largest share in 2024. Assay kits are extensively applied for detection of several cytokines such as interleukins (ILs) and interferons (IFNs), in detecting antibody-secreting cells (ASCs), memory B cells and responses of T-cells and B-cells. The rising investments for vaccine development, development of innovative platforms, surging chronic disease burden, expansion of biopharmaceutical and biotechnology industries, increased research activities on autoimmune disorders, government support, accelerated regulatory approvals and growing use of assay kits in pre-clinical and clinically regulated bioanalysis are the factors driving the market dominance of this segment.
Companies are focused on developing and launching ImmunoSpot assay kits such as T-cell kits, B-cell kits, Human NK-TVA (Natural Killer Target Visualization Assay) kits, cell counting kits and ferret antibodies which contain enzymatic or fluorescent detection reagents, diluent buffers, capture and detection antibodies, serum-free cell assay medium and PVDF-membrane plates providing all necessary sources for performing tests and diagnosis for end-users.
The analyzers segment is anticipated to witness lucrative growth during the forecast period. Analyzers combine microscopy and image analysis software for counting spots in assays with high sensitivity which can be applied in different fields such as virology, immunology, cancer research among others. These instruments are specially designed for the detection and quantification of cytokine-secreting cells or other analytes and used for analyzing microtiter plate-based assays or Peri-dish based assays, offering mutlifunctionality beyond ELISpot and FluoroSpot assays. The ongoing advancements for developing advanced and high-performance analyzers and growing use in various medical and research purposes is expected to drive the market growth of this segment in the upcoming years.
The fluorescence-enabled ImmunoSpot analyzers developed by Cellular Technology Limited (CTL) is an innovative HDR imaging platform for FluoroSpot counting applications offering optimized accuracy and speed.
Diagnostic applications segment held the largest market share in 2024. The market dominance of this segment is driven by the increased use of ELISpot and FluoroSpot assays for diagnosis for various infectious diseases such HIV, tuberculosis and other viral infections as well as for accurate detection of autoimmune disorders. Furthermore, increased demand for early disease diagnosis due to growing patient awareness, rising investments for developing novel diagnostics assays, growing emphasis on personalized medicine and ongoing advancements in oncology and immunology with enhanced sensitivity and specificity of these assays in cancer research and organ transplantation are the factors boosting the market growth.
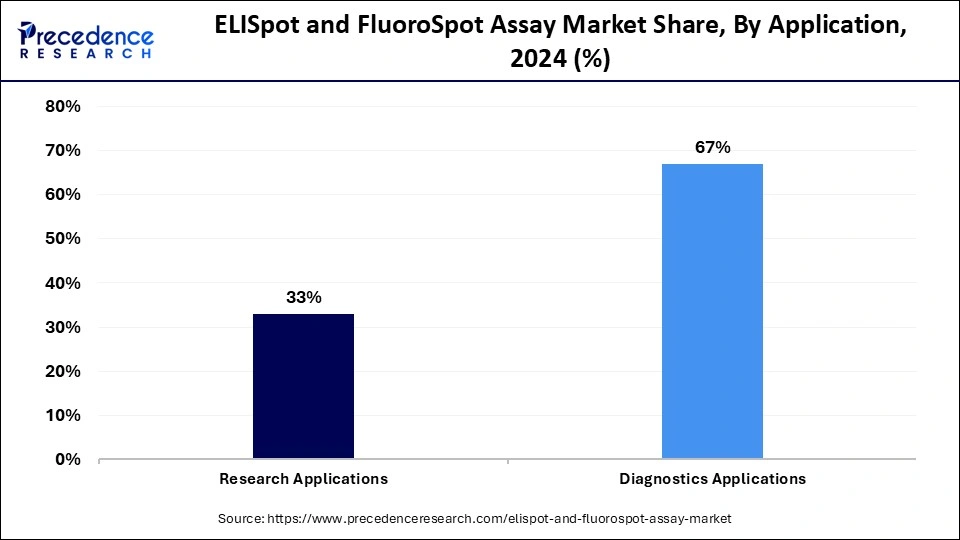
Research applications segment is anticipated to show the fastest growth during the forecast period. The growing use of ELISpot and FluoroSpot assays in various research applications such as for autoimmune disease research, in monitoring immune response to transplant antigens, in drug development for identifying potential drug candidates regulating immune responses and the multiplexing capabilities offered by these assays for simultaneously detecting multiple cytokines with the continuous advancements in assay technology enhancing the accessibility and user convenience are the factors fuelling the market growth of this segment.
Hospitals and clinical labs segment accounted for the largest market share in 2024. The rising investments by various governments across the globe advancing healthcare infrastructure and for funding research programs, surging cases of chronic and infectious diseases, growing collaborations with research institutes and biopharmaceutical industries, need for diagnostic tools with enhanced accuracy and sensitivity, increased emphasis on early disease diagnosis allowing timely interventions and growing number of clinical testing laboratories are the factors driving the market growth of this segment.
Biopharmaceutical companies segment is predicted to show the fastest growth over the forecast period. The continuous expansion of biopharmaceutical industries in various areas such as immunotherapy and vaccine development, rising chronic disease burden creating the demand for novel therapies and precision medicine with improved therapeutic potential, heavy investments by companies for advancing diagnostic and other applications, development of innovative platforms and the high specificity of these assays for measuring monocytes, B-cells, dendritic cells and antigen-specific T cells at lower frequencies in vivo as well as the accuracy of ex vivo frequency measurement for cell-mediated immune response are the factors driving the markwt growth of this segment. Furthermore, the ELISpot and FluoroSpot assays are widely used by biopharmaceutical companies in preclinical and clinical development which also includes phase 3 clinical trials owing to the high sensitivity and functionality of these assays.

By Product
By Application
By End-use
By Region
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
January 2025
February 2025
August 2024
March 2025