August 2024
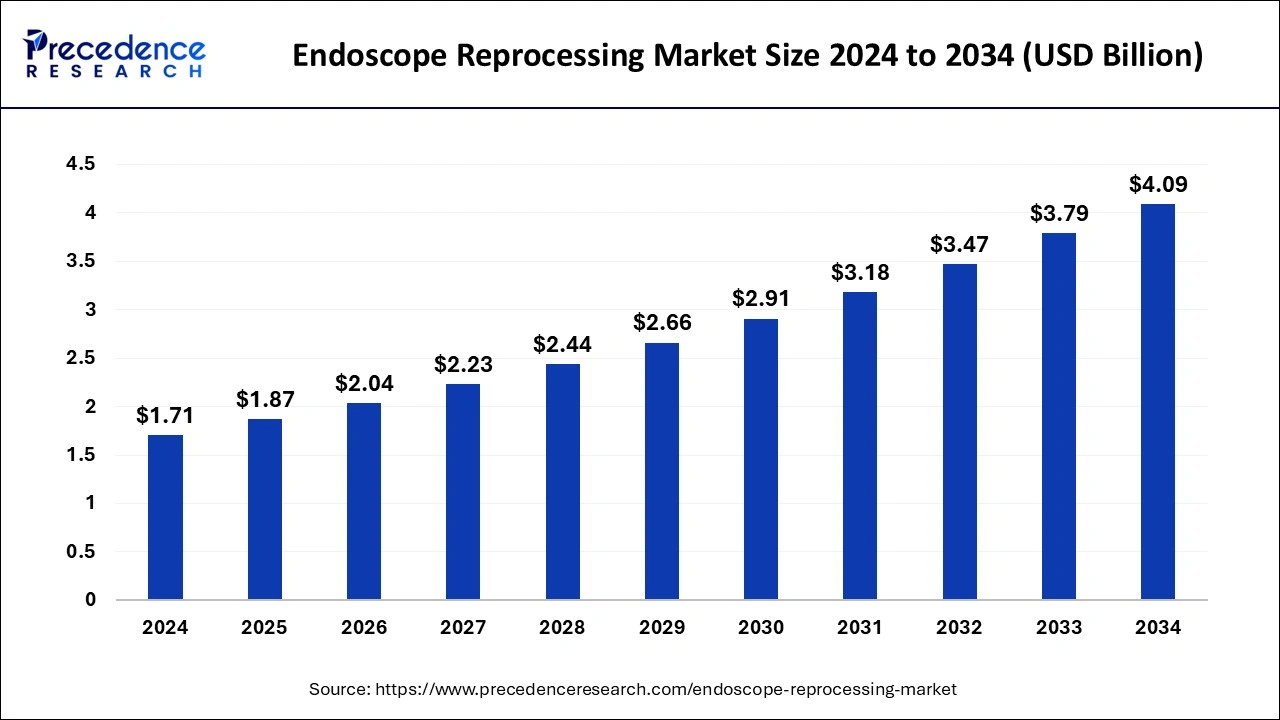
The global endoscope reprocessing market size is calculated at USD 1.87 billion in 2025 and is forecasted to reach around USD 4.09 billion by 2034, accelerating at a CAGR of 9.11% from 2025 to 2034. The North America endoscope reprocessing market size surpassed USD 700 million in 2024 and is expanding at a CAGR of 9.15% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global endoscope reprocessing market size was calculated at USD 1.71 billion in 2024 and is expected to reach around USD 4.09 billion by 2034, expanding at a solid CAGR of 9.11% from 2025 to 2034. The rising requirement for automation and patient safety in the healthcare industry is observed to boost the growth of the endoscope reprocessing market.
Cutting-edge technology, including robots, artificial intelligence (Al), and Internet of Things (IoT) integration, are used in ongoing improvements in AER systems. Al and robotics make it easier to perform accurate cleaning and disinfection procedures, reducing human error and guaranteeing complete decontamination. The performance and lifetime management of AER systems are improved by IoT integration, which makes remote monitoring, data analytics, and predictive maintenance possible. Al algorithms help to improve overall efficacy by recognizing any problems in endoscope reprocessing and optimizing cleaning procedures.
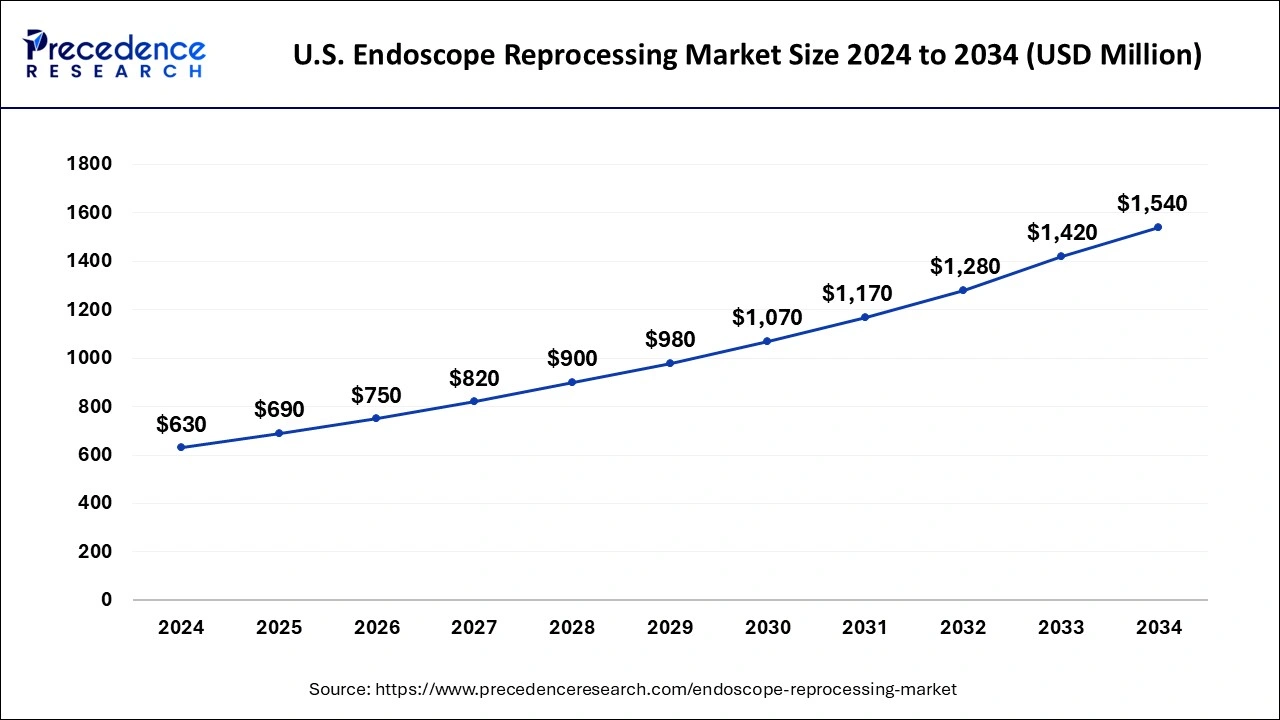
The U.S. endoscope reprocessing market size was valued at USD 630 million in 2024 and is expected to be worth around USD 1,540 million by 2034, growing at a CAGR of 9.35% from 2025 to 2034.
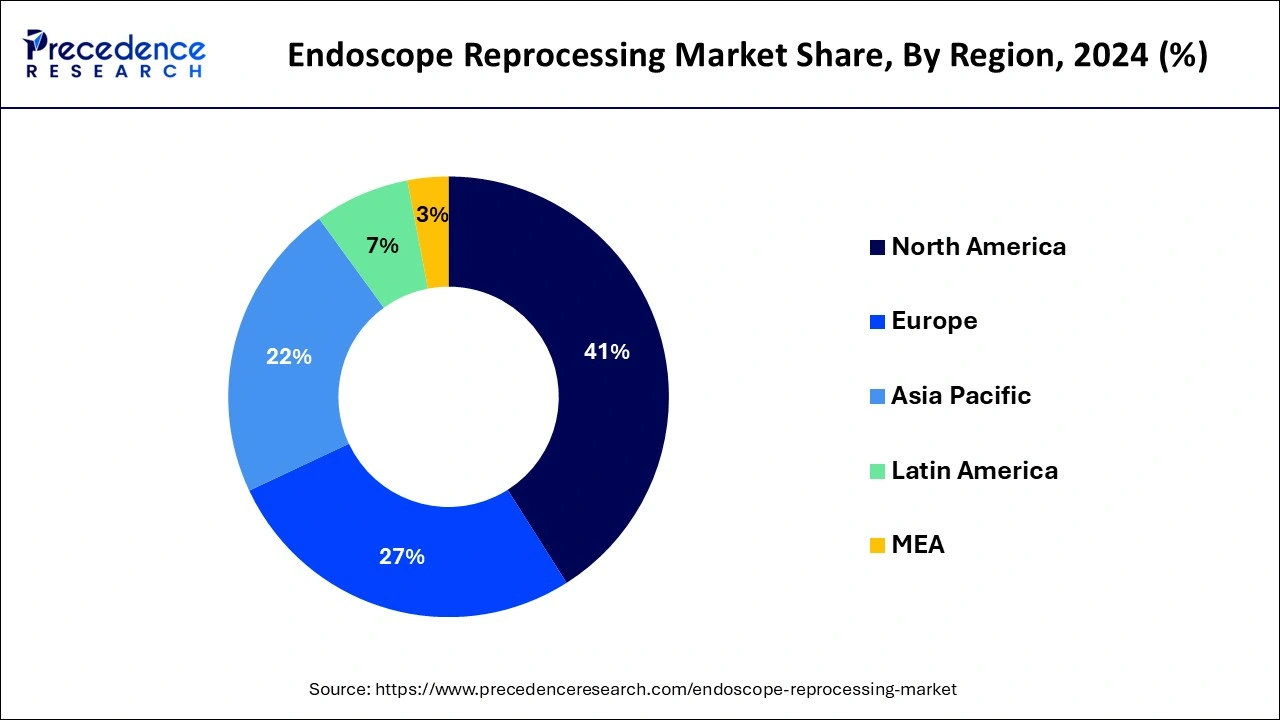
North America dominated the endoscope reprocessing market in 2024. The rising endoscope-related infections, increasing cancer rates, and beneficial reimbursement circumstances help the growth of the market in the North American region. The U.S. regulatory guidelines for endoscope processing help the growth of the market. The U.S. guidelines recommend 10 minutes for the drying cycle for endoscope reprocessing. In the United States, automated endoscope reprocessers are highly used. In Canada, the Infection Prevention and Control Guidelines for endoscope processing is a 3-stages process that helps the growth of the market. These factors help the growth of the endoscope reprocessing market in the North American region.
Endoscope reprocessing is a process of decontaminating devices widely used in the health care setting, such as endoscope accessories and duodenoscopies. The endoscope reprocessing market is driven by the increasing cases of endoscopy-related infections and increasing hospital investment in endoscopy instruments. Endoscope reprocessing includes drying, disinfecting, cleaning, and transport of endoscopes takes place between each patient. Depending on the endoscope, it also includes chemical crushing, high-heat automatic disinfection, and manual scrubbing. There are seven steps involved in endoscope reprocessing: precleaning, cleaning, rinsing, disinfection, rinsing, drying, and storage. Endoscope reprocessing advantages include it requires low initial costs, adaptability, and immediate quality checks. These factors help to the growth of the market.
| Report Coverage | Details |
| Market Size in 2025 | USD 1.87 Billion |
| Market Size by 2034 | USD 4.09 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 9.11% |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product Type, End-use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Rising requirements of the endoscopy
The rising requirement for endoscopy for the diagnosis and treatment of diseases helps the growth of the market. Endoscopy is used to help the doctor determine the cause of abnormal symptoms. It helps to see inside the body at the time of surgical procedures like repairing tumors, stomach ulcers, or removing gallstones, for the treatment of digestive tract problems. Endoscopes are helpful in illumination in one direction and high-resolution images and help to examine internal organs such as the esophagus and the throat. These factors help to the growth of the endoscope reprocessing market.
Disadvantages of the endoscope reprocessing
The disadvantages of endoscope reprocessing include its failure to dry the endoscope. It can provide an invalid reprocessing procedure, and the use of the endoscope in clinical settings leads to infection risk. There are many risks associated with the reprocessing of medical devices like endoscopy, including HAIs (Healthcare-Associated Infections), which are the major global safety concerns that may occur in medical care receiving patients and related remarkable mortality, morbidity, and healthcare costs. This leads to prolonged hospital stays and may cause accidental deaths, long-term disability, antimicrobial resistance increases, and a remarkable financial burden for the health system. In some cases, high-temperature sterilization can damage the device, and low-temperature sterilization can harm the patients and users. These factors can restrict the growth of the endoscope reprocessing market.
Research & development
There is an opportunity for endoscope reprocessing in the implementation of advanced technologies like automotive, artificial intelligence (AI), machine learning (ML), and the Internet of Things (IoT). Automated endoscope reprocessing advantages include reliability, efficiency, and consistency of endoscope reprocessing by standardizing and automizing many essential reprocessing steps to reduce human error possibilities. Automated endoscope reprocessing machines provide many different benefits over manual or hand-operated machines. Benefits of automated endoscope reprocessing include patient safety, staff safety, process standardization, facility productivity, and many financial benefits. These factors help to the growth of the endoscope reprocessing market.
The high-level disinfectants and test strips segment dominated the endoscope reprocessing market in 2024. The high-level disinfectants in the endoscope reprocessing are critical to ensure they are safe for reuse. The process of high-level disinfectant includes the steps of manual cleaning prior to high-level disinfection, high-risk device sterilization, rinsing to remove residential disinfectant, and drying to prevent bacterial proliferation and storage. The benefits of high-level disinfection include efficient cleaning and disinfecting of endoscopes by reducing cross-contamination risks and increasing operational efficiency, helping to get consistent results, increased safety for healthcare workers and patients, reducing human risk errors, helping to reduce the time required to disinfect and clean the endoscope, the healthcare facilities ensure the compliance with related guidelines and standards, and it also provides traceability and accountability.
The benefits of test strip endoscope reprocessing include automation and standardization by reducing human error and compliance with the regulatory guidelines, helping to reduce exposure of personnel to chemical sterilant or high-level disinfectant, test strips use reduces health problems associated with reprocessing among personnel, helping to reduce work-related repetitive movements which may cause injury, and also help to monitor efficiencies or low levels of hemoglobin, protein, and carbohydrates to ensure proper cleaning. These advantageous factors help the growth of the high-level disinfectants and test strips product type segment and contribute to the growth of the endoscope reprocessing market.
The automated endoscope reprocessors segment is the fastest-growing during the forecast period. The automated endoscope reprocessor helps in standardization by minimizing human errors and variations. It has a high efficiency in handling multiple endoscopes simultaneously and can complete the cycle in a shorter period than manual or traditional methods. The automated endoscope reprocessers are designed for accuracy, consistency, and cost-effective operations. The benefits of endoscope reprocessing include patient safety, staff safety, process standardization, productivity facilities, and many financial benefits. Automated endoscope reprocessors with documentation properties or features record details of each cycle, which may play a key role in quality control and compliance. It helps in safety by reducing technician contact with high-level contaminants and disinfectants from the endoscope. As compared to automated endoscope reprocessors, manual endoscope reprocessing may compromise the effectiveness of reprocessing because of susceptible inconsistencies due to human errors, is more time-consuming than the automated endoscope processing, less effective with high endoscope use, technicians may be more exposed to the disinfectants, which leads to health risk. These factors help the growth of the automated endoscope reprocessors product type segment and contribute to the growth of the endoscope reprocessing market.
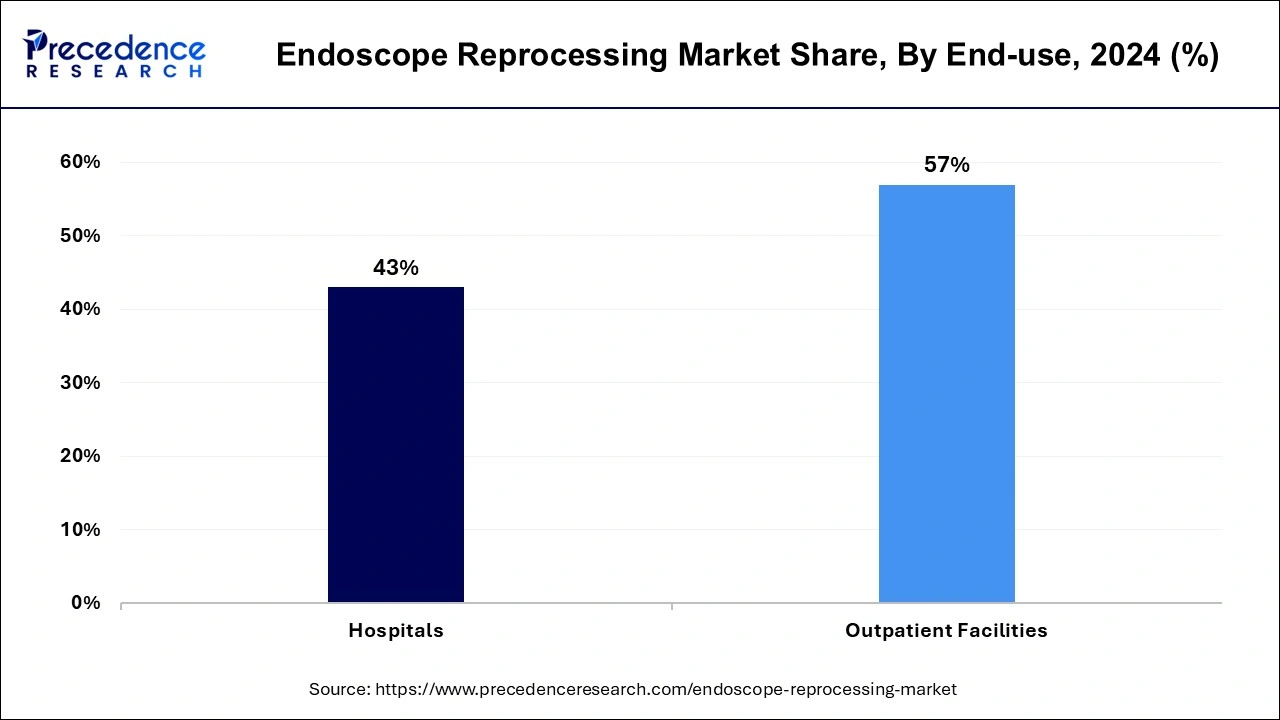
The outpatient facilities segment dominated the endoscope reprocessing market in 2024. For outpatient end-use type facilities, endoscope reprocessing is used which includes ambulatory surgical centers (ASCs). Ambulatory surgical centers (ASCs) are outpatient end-use types of facilities where many medical procedures, including endoscopic and colonoscopy procedures, are performed. These procedures help to diagnose and treat digestive system conditions, such as cancers, bleeding, polyps, ulcerative colitis, and Crohn’s disease. The benefits of endoscope reprocessing include infection prevention by eliminating or removing transmission of pathogens to patients undergoing endoscopy, patient safety proper reprocessing ensures that the endoscopes are safe for successive use by minimizing infection risks.
Ambulatory surgical centers looking for certification and maintaining accreditation should follow reprocessing guidelines to prevent infection transmission and protect patients. An authentic reprocessing program with trained staff ensures consistent safety and quality. Endoscope reprocessing is used in ambulatory surgical centers (ASCs) to maintain high-quality care and patient protection. These factors help to grow the outpatient facilities end-use type segment and contribute to the growth of the endoscope reprocessing market.
The hospitals segment is the fastest-growing during the forecast period. The use of endoscope reprocessing in hospitals has many benefits, including infection control and patient safety, time efficiency, financial benefits, and reduced risk of damage. The proper endoscope reprocessing ensures that these types of medical devices are safe for patient use. By following the necessary steps, healthcare facilities may minimize infection risks that are linked with the endoscopes. Single-use endoscopes contribute to better patient safety by reducing the risk of patient-to-patient infection transmission. The use of an automated endoscope reprocessing is high. A large number of cases can be scheduled and processed each day, which results in higher profitability for healthcare facilities. Automated endoscope reprocessing helps to increase productivity and reduce the handling of the endoscope at the time of processing, helping to prevent damage. Endoscope reprocessing may be time-consuming. By using effective endoscope processing methods, healthcare staff or doctors may redirect their time toward the patient’s care. It also reduces the risk of damage and ensures consistent performance. These factors help the growth of the hospital end-use type segment and contribute to the growth of the endoscope reprocessing market.

By Product Type
By End-use
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
August 2024