List of Contents
Medical Device Clinical Trials Market Size and Forecast 2025 to 2034
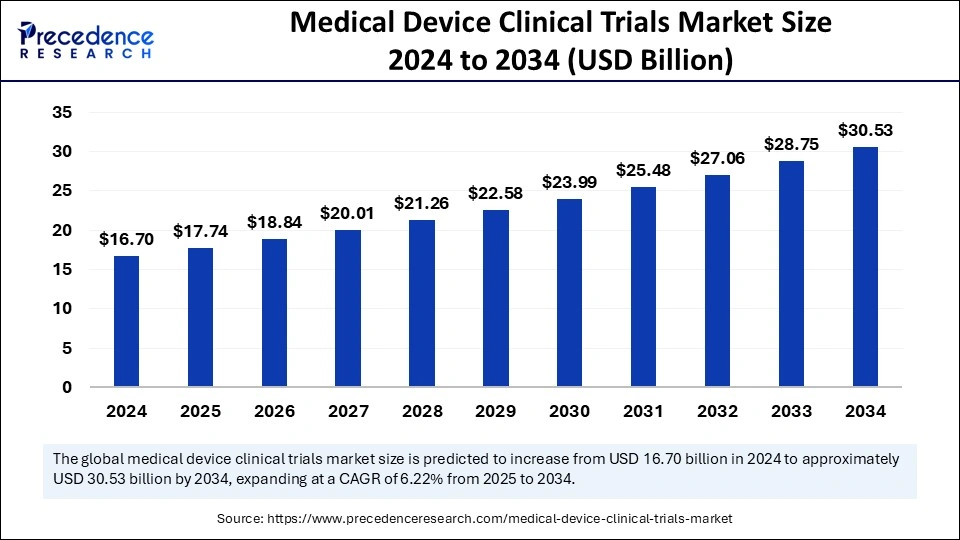
The global medical device clinical trials market size was calculated at USD 16.70 billion in 2024 and is predicted to increase from USD 17.74 billion in 2025 to approximately USD 30.53 billion by 2034, expanding at a CAGR of 6.22% from 2025 to 2034. The demand for medical device clinical trials is increasing due to the rising prevalence of chronic disease, which is driving advancements in the healthcare industry.
Medical Device Clinical Trials Market Key Takeaways
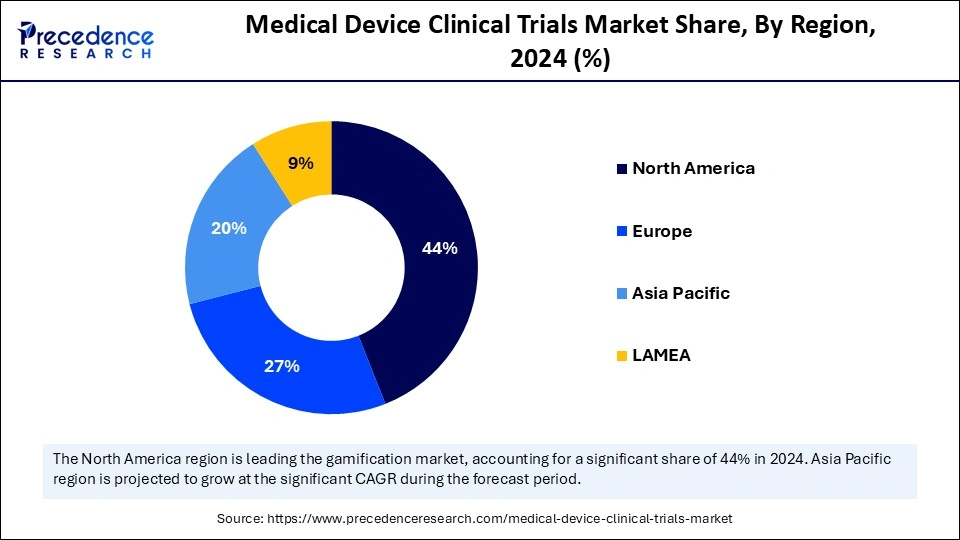
- North America dominates the global market by generating the largest share of 44% in 2024.
- Asia Pacific is anticipated to emerge as the fastest-growing market during the forecast period of 2025 to 2034.
- By study type, the pivotal study segment dominated the global market in 2024.
- By study type, the feasibility and pilot study segment is anticipated to emerge as the fastest-growing during the forecast period of 2025 to 2034.
- By study design, the interventional segment held the largest market share in 2024.
- By study design, the observational segment is anticipated to grow at the highest CAGR during the forecast period of 2025 to 2034.
- By indication, the cardiovascular devices segment marked its dominance by contributing to the largest share in 2024.
- By indication, the neurology segment is expected to grow at a CAGR of 7.4% during the forecast period of 2025 to 2034.
How are Artificial Intelligence (AI) and Machine Learning (ML) Changing the Medical Device Clinical Trials Market?
The emergence of technologies like Artificial Intelligence (AI) and Machine Learning (ML) are playing a transformative role in the healthcare industry. The use of AI is increasing due to its algorithm which helps in analyzing patient data during clinical studies. This helps in reducing errors during the procedures and also maintains the engagement of the patient participation in these studies.
The companies are using AI models in trial designs, which enables real-time modification of the resources. Additionally, the data analyzing and storing capacity also makes it ideal for the companies which can further be useful in the future too. The rising technological advancements are also helping in targeting personalized trials for various categories and age groups. These technologies are applying critical thinking which is helping in reducing the human time consumed during the trials.
U.S. Medical Device Clinical Trials Market Size and Growth 2025 to 2034
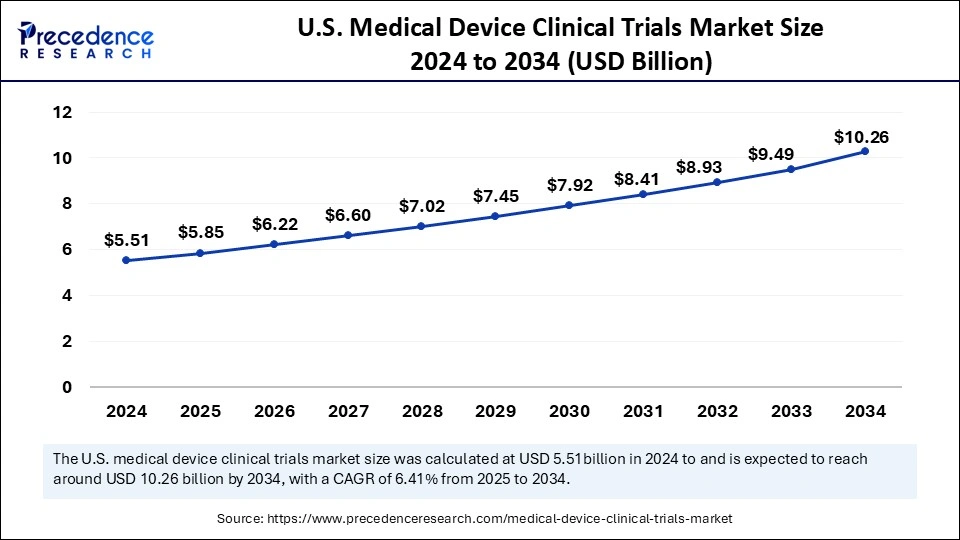
The U.S. medical device clinical trials market size was exhibited at USD 5.51 billion in 2024 and is projected to be worth around USD 10.26 billion by 2034, growing at a CAGR of 6.41% from 2025 to 2034.
North America dominated the global medical device clinical trials market by generating the largest revenue share in 2024. The dominance of the region is attributed to the strong presence of the FDA, which supports approval at a faster pace. The companies in the region are also expanding as the governments are focusing on the advancement of healthcare services.
The stronger presence of the United States is playing a crucial role in the region's demand where the country is focusing on the expansion of clinical studies. The technological presence in the country is the main reason behind the growth of the market in the region. The adoption of AI in the U.S. healthcare industry is anticipated to help the country grow more rapidly in the upcoming years.
Asia Pacific is anticipated to emerge as the fastest-growing market during the forecast period of 2025 to 2034. The growth of the region is attributed to the rising urbanization in countries like China, Japan, and India. The rising urbanization is also contributing towards chronic disease prevalence which further drives demand for medical devices.
China stands out to be the dominant player in Asia Pacific due to its Made in China 2025 initiatives that focus on improving medical innovation in the country. The country is also experiencing investments in the clinical infrastructure, creating business opportunities for companies. Additionally, the lower manufacturing costs in the country are expected to help the country grow more efficiently in the coming years.
Market Overview
A medical device clinical trial is a study mainly conducted to evaluate the performance and safety of medical devices before their approval. Various organizations are involved in the process, and they ensure the regulatory standards are fulfilled by the devices. The medical device clinical trials market is growing rapidly due to the growing popularity of advanced equipment in the healthcare industry, which mainly focuses on patient safety and service outcomes. There are various processes like non-clinical studies, pilot studies, pivotal studies, and post-market surveillance that use various patients for their clinical purposes.
Medical Device Clinical Trials Market Growth Factors
- The rising prevalence of chronic diseases in developing countries is creating demand for medical devices for diabetes, CAD, and neurological disorders.
- The rising geriatric population is also playing a crucial role in the market demand as the increasing patient volume helps in the growth of clinical trials.
- The rising government funding for R&D is playing a transformative role as it is creating business opportunities for these medical device companies.
- The growth of personalized medicines is a major factor that drives the demand for clinical trials where companies adopt advanced technologies for enhanced outcomes.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 30.53 Billion |
| Market Size by 2025 | USD 17.74 Billion |
| Market Size by 2024 | USD 16.70 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 6.22% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2024 to 2034 |
| Segments Covered | Study Type, Study Design, Indication, and regions |
| Regions Covered | America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Market Dynamics
Drivers
R&D in innovative devices
The rising prevalence of diseases has been attracting attention to healthcare solutions that can help individuals. Healthcare device manufacturers are producing technological advancements like bioelectronic devices, AI diagnostics, and many others. This is raising the clinical trial frequency and raising the demand for more efficient solutions where rising investments are helping in the enhanced outcomes. These R&Ds are helping the companies to grow in the competitive business environment where they can grab easy approvals from organizations. For instance, da Vinci's Surgical System is an R&D-driven robotic-assisted surgical system that helps in increasing patient safety and medical outcomes.
Growth of wearable devices
Rapid urbanization is leading towards many changes in lifestyles where individuals are focused on improving their overall health. The use of wearables is increasing as it helps in tracking the day-to-day activities of the individuals involved in clinical trials. The medical device clinical trials market is growing rapidly where the companies are focusing on these devices which can provide insights into heart irregularities, glucose fluctuations and others. The rising focus on the improvement of these devices is driven by their growing prevalence. The growth of implantable devices is also playing a crucial role in these clinical trials.
Restraint
Higher clinical trial costs
The rising patient volume in developing and underdeveloped countries is leading towards increasing requirements for advanced healthcare solutions. This is leading towards increasing R&D in clinical trials for medical devices and medicines. The medical device clinical market could face certain challenges in underdeveloped countries due to their budgetary limitations, which makes patient recruitment, approval, and other things unaffordable. Additionally, the imported devices are also higher in cost which increases the overall healthcare costs in the region. The unavailability of advanced technologies in these regions is also anticipated to play a restraining role in the market.
Opportunity
Increasing stricter regulations
The increasing patient volume is leading towards an increasing focus on organizations that ensure patient safety and successful medical outcomes. The medical device clinical trials market is expected to rise rapidly as the companies are expected to conduct more trials due to the strict implementation by the FDA (United States, CDSCO (India) and EMA (Europe). These companies are also anticipated to invest heavily due to the rising market competitiveness that would help them attract more revenue. These initiatives are also creating a business market for regulatory consultants who help medical device manufacturing companies.
Growing demand for virtual trials
Rapid digitalization is one of the major factors that is leading towards significant changes in the healthcare industry. Healthcare companies are using these technologies to execute these trials which is eliminating the physical patient requirement during these processes. The medical device clinical trials market is expected to rise rapidly as virtual trials eliminate additional costs. Technological advancements are also anticipated to reduce the time consumed during these processes. The adoption of virtual trials is also eliminating geographical barriers which can focus on more accurate results.
Study Type Insights
The pivotal study segment stood dominant by generating the highest share in 2024. These are final evidence trials performed on a large scale, which mainly ensure the safety and performance before the approval. The medical device clinical trials market is growing rapidly, and demand for regulatory approvals is increasing rapidly. The manufacturing companies are making heavy R&D investments in pivotal trials which ensures their approval from the organizations further. The global presence of these trials is increasing which is anticipated to maintain the demand for patients in these tests. Additionally, the organizations are also increasing the approval rates due to the rising patient volume.
The feasibility and pilot study segment is expected to grow at the highest CAGR during the forecast period of 2025 to 2034. These studies are executed at an early stage, which ensures the evaluation of the functionality and initial performance of the devices. The medical device clinical trials market is growing rapidly due to the rising market competitiveness which is leading towards the innovation of new devices. The companies are anticipated to execute more studies, which would raise the requirement for feasibility and pilot studies. The rise of technologies is helping companies to gain more accurate results during procedures.
Study Design Insights
The interventional segment held the highest market share in 2024. These trials are executed where the researcher or professional assigns specific treatment to the participant. They use various methods like randomized controlled trials and single-arm studies which cover various treatments and tasks. The medical device clinical trials market is growing rapidly, and the safety standards make these trials effective. The companies are investing in devices like pacemakers, robotic surgical systems, stents and many others that would increase the demand for interventional trials in the future too.
The observational segment is anticipated to grow at the highest CAGR during the forecast period of 2025 to 2034. These studies focus on analyzing real-time data of the patients already using medical devices. The medical device clinical trials market is growing rapidly and the regulations are mandating post-market surveillance that would ensure patient safety in the longer run. The growth of implantable devices and smart watches contributes to these studies as they help them in constant observations. The increasing use of electronic health records in virtual trials is anticipated to boost the demand in the coming years, too.
Indication Insights
The cardiovascular disease segment stood dominant with the highest share in 2024. The dominance of the segment is attributed to the rising death rates because of these diseases. The companies are focusing on innovating devices for heart conditions like CAD, heart failure, etc. The medical device clinical trials market is growing rapidly as governments are focusing on improving healthcare solutions through improving these devices. The rising prevalence is also mandating the use of these devices in public and private hospitals.
The neurology segment is anticipated to grow at the highest CAGR during the forecast period of 2025 to 2034. The rising prevalence of Alzheimer's and Parkinson's disease is leading towards investments that advance deep brain stimulators and other devices. The medical device clinical trials market is anticipated to grow rapidly due to the use of AI in neuroimaging which would enhance the medical outcomes for brain-related diseases. The rising prevalence is also leading towards the growing focus of startups that are researching brain disorder solutions.
Medical Device Clinical Trials Market Companies

- Medtron ic
- Siemens Healthineers AG
- Fresenius Medical Care AG
- GE Healthcare
- Koninklijke Philips N.V.
- Danaher Corporation
- Baxter
- Boston Scientific Corporation
- F. Hoffmann La Roche
Announcements by Industry Leaders
- In March 2025, Prof. Dr. Med. Dent. Patrick Schmidlin, head of the Division of Periodontology at the University of Zurich, commented on RevBio's clinical trial, stating, “The results from this ongoing international multicenter study are exceeding expectations, showcasing remarkable outcomes. This breakthrough material promises to open a new chapter in dental care, redefining what is possible in regenerative dentistry.”
- In February 2025, the University of Virginia launched a clinical trial to test an AI-powered device for automated insulin delivery in Type 1 Diabetes management, aiming to simplify treatment and improve blood sugar control. "We are committed to creating a fully automated, intelligent insulin delivery system that redefines diabetes management, making treatment simpler, more reliable, and entirely effortless for patients," said Heman Shakeri, Assistant Professor of Data Science at the University of Virginia.
Recent Developments
- In February 2025, the University of Virginia launched a clinical trial to test an AI-powered device for automated insulin delivery in Type 1 diabetes management
- In March 2025, Lindus Health and Sooma Medical announced a phase 3 clinical trial to evaluate the effectiveness of Sooma's transcranial direct current stimulation (tDCS) device, Sooma 2GEN, for treating major depressive disorder (MDD).
Segments covered in the report
By Study Type
- Feasibility and Pilot Study
- Pivotal Study
- FDA PMA Application
- Post-Approval Study
By Study Design
- Interventional
- Observational
- Expanded Access
By Indication
- Cardiovascular devices
- Neurology devices
- Orthopedic devices
- Diagnostic imaging
- Anesthesia and Respiratory devices
- Others
By Region
- North America
- Latin America
- Europe
- Asia-pacific
- Middle and East Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client