February 2025
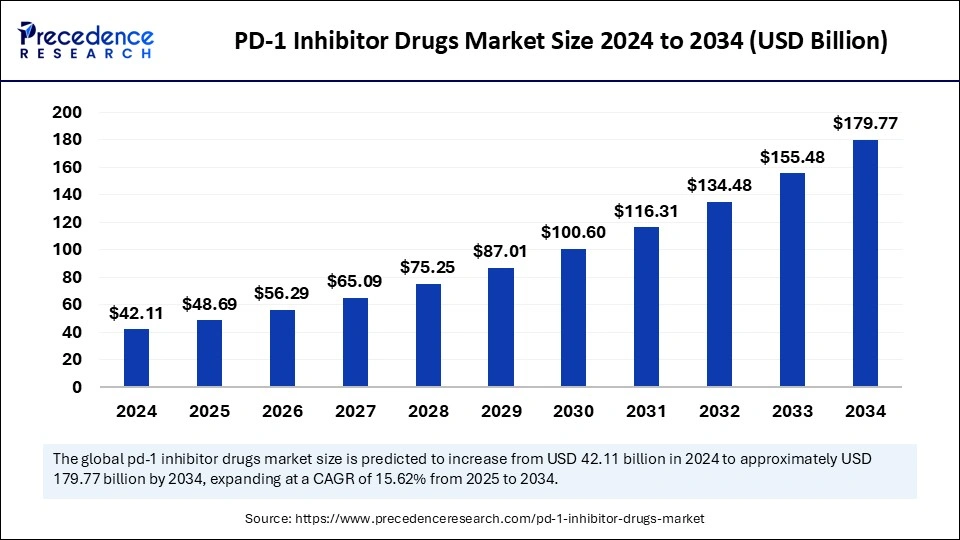
The global PD-1 inhibitor drugs market size is calculated at USD 48.69 billion in 2025 and is forecasted to reach around USD 179.77 billion by 2034, accelerating at a CAGR of 15.62% from 2025 to 2034. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global PD-1 inhibitor drugs market size accounted for USD 42.11 billion in 2024 and is predicted to increase from USD 48.69 billion in 2025 to approximately USD 179.77 billion by 2034, expanding at a CAGR of 15.62% from 2025 to 2034. The market is driven by rising cancer incidence, expanding regulatory approvals, advanced combination therapeutic approaches, and improved accessibility to innovative immunotherapy.
PD-1 inhibitor drugs market development and their clinical applications benefit from artificial intelligence technology leading to faster innovation within cancer immunotherapy. The modern AI tools process large data collections from clinical trials in addition to genomic studies and patient records to discover biomarkers. AI technology enables scientists to identify key genetic and molecular signatures, which leads to precision medical treatment development for patients.
AI diagnostic technology helps oncologists use data-driven methods during cancer treatment while they improve treatment plans by monitoring patient responses. AI technology makes the clinical trial process more efficient through the examination of the most suitable candidates, adverse event forecasting, and real-time patient information analysis.
The PD-1 inhibitor drugs operate as checkpoint inhibitors by blocking the PD-1 pathway to stop cancer cells from hiding from immune system detection. PD-1 inhibitor drugs revolutionize oncological treatment by stimulating T-cells to target tumors, thus benefiting patients with non-small cell lung cancer (NSCLC), melanoma, head and neck squamous cell carcinoma (HNSCC), and other cancers. These therapies continue to transform clinical practices globally because they improve treatment survival and maintain response durations.
A robust expansion applies to the PD-1 inhibitor drugs market because of growing global cancer incidence and healthcare providers adopting immunotherapy as an initial treatment choice. The market experiences growth due to regulatory agencies approving new treatment areas, drug label expansions, and promising results from combination therapy studies. The market expansion stems from cancer screening advancements as well as growing errors involving biomarker-based medical treatments.
| Report Coverage | Details |
| Market Size by 2034 | USD 179.77 Billion |
| Market Size in 2025 | USD 48.69 Billion |
| Market Size in 2024 | USD 42.11 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 15.62% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Drug Type, Indication, Distribution Channel, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Rising cancer incidence and growing adoption of immunotherapy
The market expands due to increasing cancer rates alongside the growing usage of immunotherapy treatment. Cancer stands as one of the main killers worldwide, while development rates of lung cancer, melanoma, head and neck cancers, and other forms continue to increase. The rising number of cancer cases requires new treatments that deliver superior treatment benefits and safer outcomes than conventional therapeutic methods.
PD-1 inhibitor drugs inhibit PD-1 protein actions to allow the immune system to identify cancer cells. Healthcare providers currently administer PD-1 inhibitors as standalone therapy or in combination regimens after drug makers obtain positive trial results and receive expanded treatment approvals from regulators.
High treatment costs and limited accessibility
High prices of PD-1 inhibitor drugs create the primary market obstacle that prevents patients from lower- and middle-income countries from accessing these medications. PD-1 inhibitors Keytruda and Opdivo generate significant healthcare expenses which produce unfavorable impacts on patient spending and institution resources. Permanent financial pressure exists in healthcare systems due to prolonged therapy with immunotherapy medications. Several factors, such as strict regulations, production complexities, development protocols, and advanced manufacturing processes, result in the high costs.
Rising potential for combination therapies
The PD-1 inhibitor drugs market expands because researchers implement synergistic treatment approaches by combining drugs with chemotherapy and targeted therapies or other immune checkpoint inhibitors. Partnerships between these drugs create treatments that enhance effectiveness and enable patients to exhibit better outcomes and increased survival rates. The market needs to continue to drive additional growth as health authorities give approvals to new treatment options. The pharmaceutical industry is an excellent space to establish innovative treatment approaches leading to superior clinical success for more patients.
The pembrolizumab segment contributed the largest share of the PD-1 inhibitor drugs market in 2024. Humanized monoclonal antibody pembrolizumab interrupts PD-1 signaling with PD-L1 and PD-L2 molecules to allow cancer-detecting functions of the immune system. Medical professionals use pembrolizumab (Keytruda) to treat non-small cell lung cancer (NSCLC), melanoma, head and neck squamous cell carcinoma, as well as Hodgkin lymphoma.
Pembrolizumab dominates the market because of its expanded medical applications across multiple cancers, with evidence showing extended patient survival and better results. The successful use of pembrolizumab created a benchmark standard for PD-1 inhibitors, which leads to its status as a vital component of modern cancer treatment.
The nivolumab segment is expected to show considerable growth in the forecast period. Nivolumab functions as a monoclonal antibody, which stops PD-1 from blocking T-cell activation, thus allowing cancer cells to get eliminated more efficiently. The drug finds application in treating multiple cancer types, including melanoma, renal cell carcinoma, Hodgkin lymphoma, and esophageal cancer. Worldwide, medical institutions have adopted Nivolumab due to its proven effectiveness against challenging cancers when administered either as an adjuvant or in metastatic stages.
The combination of nivolumab with ipilimumab (Yervoy) through targeted therapies has proven effective in improving patient survival rates and diminishing cancer advancement. The nivolumab segment will experience significant market growth because of improving healthcare accessibility and rising global cancer incidence.
The non-small cell lung cancer segment dominated the PD-1 inhibitor drugs market in 2024. The large incidence rates of lung cancer worldwide support the development of PD-1 inhibitors because these drugs demonstrate significant benefits in patient response. Many patients receive pembrolizumab and nivolumab medications that function by blocking PD-1 protein, which enables T-cell immune checkpoints to restrict attacks on healthy cells. The future market leadership of the PD-1 blocker segment seems certain because active clinical trials investigate early NSCLC applications with these drugs and combination treatments.
The melanoma segment is anticipated to witness significant growth over the studied period. The treatment landscape of melanoma experienced a breakthrough with Keytruda (Pembrolizumab) and Opdivo (Nivolumab), which strengthen the immune system to fight and destroy cancer cells. The medical drugs block PD-1 protein activity because this protein serves to slow down T-cell attacks against all cells, including tumor cells.
The blocked inhibition through PD-1 inhibitor treatment enables the immune system to launch an intensified and continuous battle against melanoma cells. The market for immunotherapies in the treatment of melanoma will expand significantly due to increasing patient access and the worldwide rise in melanoma cases.
The hospital's pharmacies segment contributed the largest share of the PD-1 inhibitor drugs market in 2024. The presence of advanced healthcare infrastructure and skilled medical professionals operates as the main driving force. The hospital pharmacy enables life-saving pharmaceutical supplier procurement of PD-1 inhibitors through approved pharmaceutical providers to maintain uninterrupted medical supplies.
The hospitals enable complete cancer treatment operations, including diagnostic services, infusion therapies, and continued patient monitoring. Thus, patients demonstrate enhanced treatment commitment and care affirmation. Outpatient care growth drives hospitals to enhance their pharmacy services for home-based care patients, thus increasing the market strength of this segment.
The online pharmacies segment is expected to show considerable growth in the forecast period. The market growth continues because patients adopt digital healthcare technologies and find more convenience in receiving medications through home delivery services. Online pharmacies provide remote and underserved locations with pharmaceutical treatments through their accessible services. The online platforms implement safe delivery systems that provide optimal drug storage to maintain PD-1 inhibitor effectiveness.
Many online pharmacies offer patient support services that provide scientific information about drug processes, related side effects, and proper dosage methods to enhance treatment compliance. Online pharmacies can safely deliver immunotherapy drugs via their channels worldwide because of enhanced logistics and strict cold chain management operations with strong regulatory compliance practices.
North America PD-1 Inhibitor Drugs Market Trends
North America accounted for the largest share of the PD-1 inhibitor drugs market in 2024 because of the region's well-developed healthcare system, large R&D expenditures, and widespread immunotherapy adoption. The clinical trial activity in this area with major pharmaceutical industry presence speeds up the process of PD-1 inhibitor approval. Effective immunotherapy demand continues to rise in the region because it serves an aging population and increases cancer incidence rates.
United States leadership stems from its vast healthcare system and the high incidence of cancer within the country. The United States remains at the forefront of PD-1 inhibitor research because scientists perform substantial clinical trials to broaden the range of cancer types treated by these drugs and refine multi-drug treatment protocols.
The American Cancer Society predicts that the United States will see these projected melanoma statistics in 2025.
Asia Pacific PD-1 Inhibitor Drugs Market Trends
Asia Pacific is anticipated to witness the fastest growth in the PD-1 inhibitor drugs market during the forecasted years. The growing cancer incidence and rising adoption of immunotherapy drugs throughout the region created this demand. Significant government funding with expanded healthcare facilities has accelerated the market introduction of PD-1 inhibitors. The market expands because patients accept immunotherapies due to increased public understanding regarding their effectiveness.
China’s elevated healthcare funding and government support of drug discovery initiatives have developed. Healthcare developments and expanded insurance coverage by the government make immunotherapies available to more patients across the region.
Europe PD-1 Inhibitor Drugs Market Trends
Europe holds a significant presence in the global PD-1 inhibitor drugs market due to its advanced healthcare systems, excellent research capabilities, and rising acceptance of immunotherapy. The government support programs and manufacturers achieve better access to new medical developments for patients. Europe continues to dominate PD-1 inhibitor research and development for multiple cancer types because it has major pharmaceutical organizations and effective collaborative research partnerships.

By Drug Type
By Indication
By Distribution Channel
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
February 2025
January 2025
March 2025
March 2025