February 2025
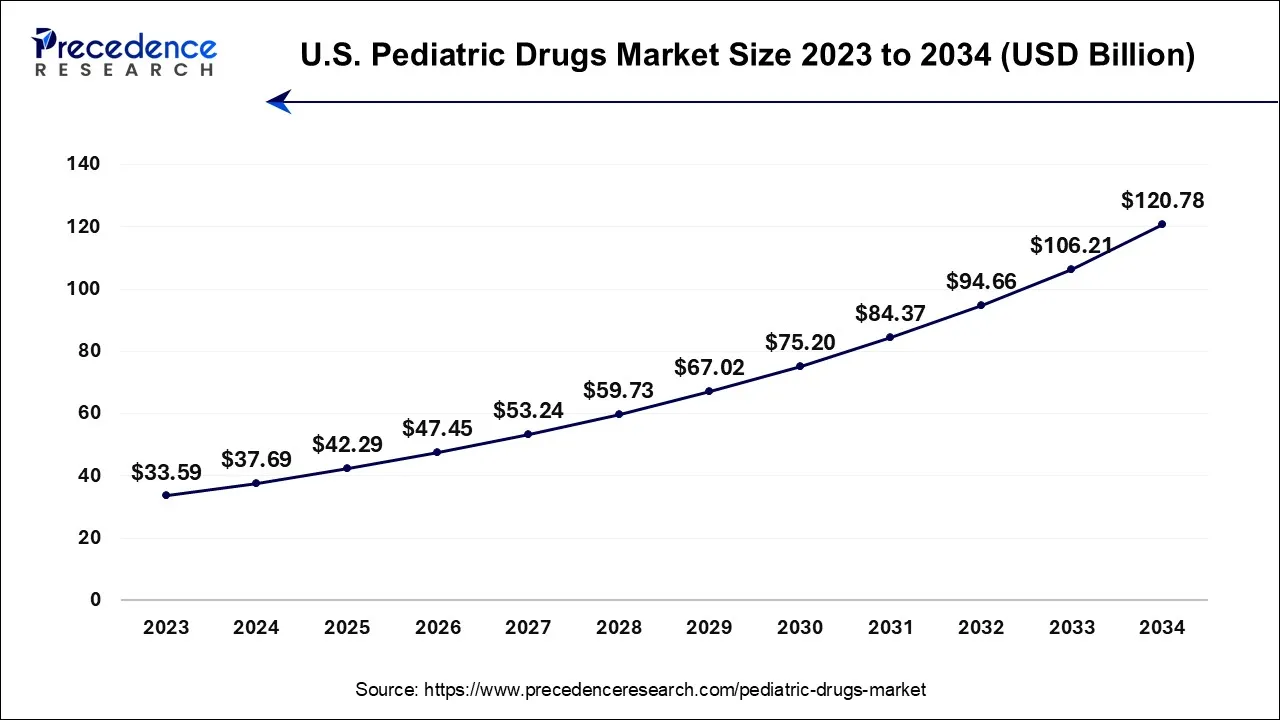
The global pediatric drugs market size is calculated at USD 145.53 billion in 2024, grew to USD 163.28 billion in 2025 and is predicted to hit around USD 460.12 billion by 2034, expanding at a CAGR of 12.20% between 2024 and 2034. The North America pediatric drugs market size accounted for USD 53.85 billion in 2024 and is representing a notable CAGR of 12.35% during the forecast period.
The global pediatric drugs market size was estimated at USD 145.53 billion in 2024 and is projected to hit around USD 460.12 billion by 2034, registering a CAGR of 12.20% from 2024 to 2034.
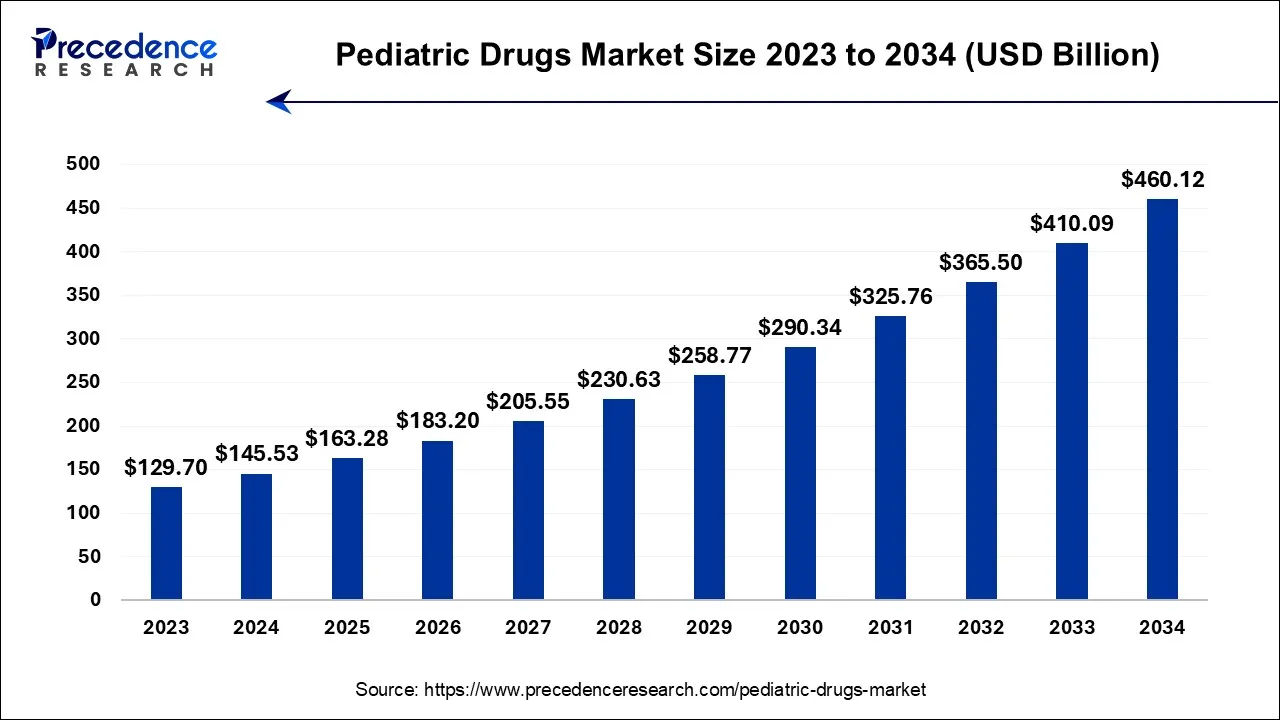
The U.S. pediatric drugs market size was exhibited at USD 37.69 billion in 2024 and is estimated to reach around USD 120.78 billion by 2034, growing at a CAGR of 12.36% from 2024 to 2034.
North America has held the largest revenue share 37% in 2023. North America's dominance in the pediatric drugs market is driven by several key factors. The region boasts a robust healthcare infrastructure, extensive research and development capabilities, and a significant pediatric patient population. Additionally, favorable regulatory frameworks and government incentives encourage the development and availability of pediatric medications. A strong focus on pediatric healthcare needs and a high level of awareness among parents and healthcare professionals further contribute to the market's growth. North America's economic strength and the presence of leading pharmaceutical companies make it a prominent player in the global pediatric drugs market.
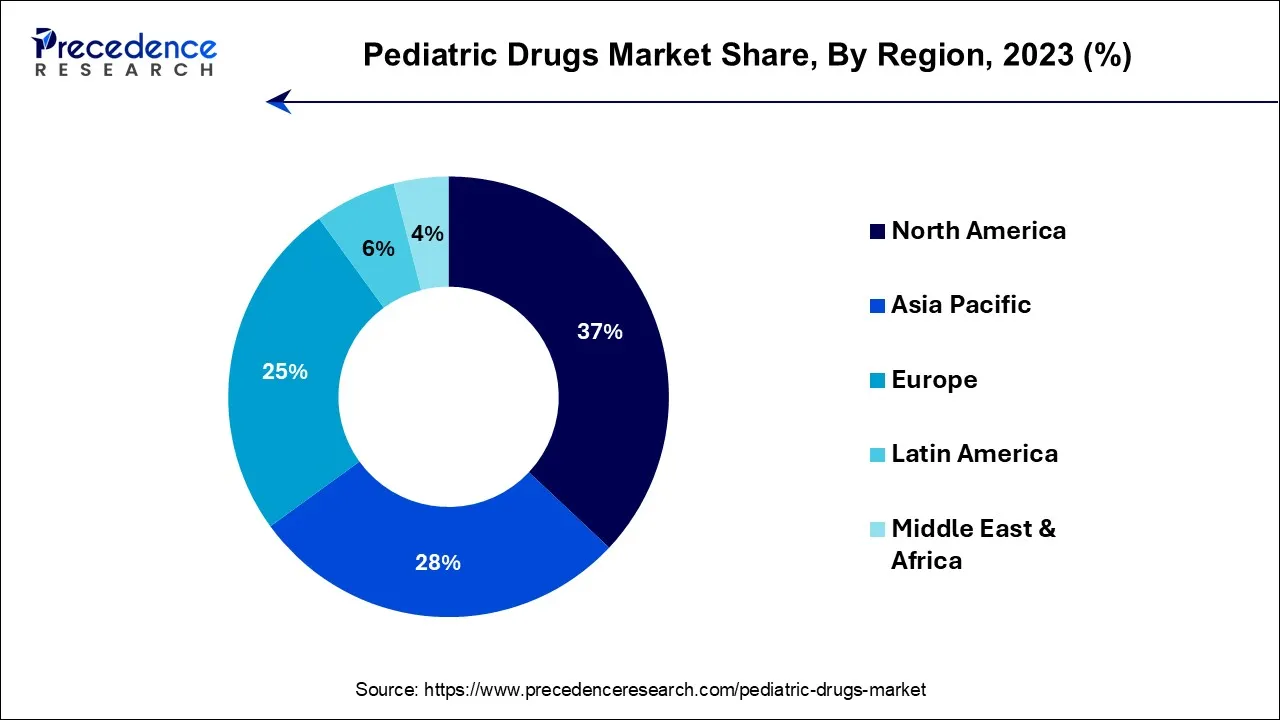
Asia-Pacific is estimated to observe the fastest expansion with the highest CAGR of 18.3% during the forecast period. The Asia-Pacific region's substantial share in the pediatric drugs market can be attributed to several pivotal factors. Firstly, the region is home to a vast and growing pediatric population, thereby necessitating an increased demand for healthcare solutions tailored to children. Furthermore, Asia-Pacific's economic prosperity and an expanding middle-class demographic contribute to improved affordability and accessibility to pediatric medications.
Government initiatives and augmented healthcare investments have further bolstered the market's expansion. Notably, the pharmaceutical industry in this region is dedicating more resources to developing medications specifically designed for pediatric patients. This collective dynamic has positioned Asia-Pacific as a prominent influencer in the pediatric drugs market, serving as a central hub for pediatric pharmaceutical research, development, and distribution.
The pediatric drugs market encompasses the pharmaceutical industry's specialized focus on the development and production of medications tailored exclusively for the pediatric population, spanning from neonates to adolescents. This market revolves around addressing the distinctive medical requirements of children, offering solutions for various pediatric conditions, including common childhood illnesses, chronic ailments, and developmental disorders.
Notably, this sector adheres to stringent regulatory standards to ensure the safety and effectiveness of drugs designed for this vulnerable patient group. Driving factors behind this market include the escalating incidence of pediatric health issues, heightened awareness of children's healthcare needs, and government-driven incentives to promote the research and development of pediatric drugs. The pediatric drugs market fulfills a crucial role in preserving and enhancing the well-being of our society's youngest members.
The pediatric drugs market forms a distinct niche within the pharmaceutical sector, focusing on the development and production of medications exclusively tailored to meet the medical requirements of infants and children. This specialized market, serving pediatric patients from newborns to adolescents, caters to a diverse range of health conditions, spanning from common childhood illnesses to intricate chronic diseases and developmental disorders. To ensure the safety and efficacy of drugs designed for this vulnerable patient group, stringent regulatory standards are meticulously upheld.
The pediatric drugs market exhibits noteworthy trends and growth-driving factors. The mounting prevalence of pediatric health challenges, encompassing issues like childhood obesity, diabetes, and neurological disorders, is stimulating a heightened demand for specialized pharmaceuticals.
The growing awareness surrounding the significance of children's health and the profound, long-term effects of early medical interventions is further propelling market expansion. In addition, governmental incentives and regulatory initiatives are fostering research and development endeavors in the formulation of pediatric medications.
The pediatric drugs market's growth is propelled by various factors, including advancements in pediatric research, the widening scope of clinical trials, and augmented investment in the development of medications specifically tailored for children. Significant business opportunities are emerging for pharmaceutical firms and biotechnology enterprises specializing in pediatric drug formulations, as well as for companies engaged in contract manufacturing services for pediatric pharmaceuticals. Collaborative research ventures, innovative drug delivery methodologies, and partnerships with healthcare providers also present promising avenues for market growth.
Despite the promising prospects, the pediatric drugs market faces certain challenges, such as ethical dilemmas linked to conducting clinical trials on pediatric subjects and navigating the intricacies of regulatory procedures. Crafting pharmaceuticals designed for pediatric use can pose technical complexities, requiring precise dosing and the creation of palatable formulations. Furthermore, the limited market exclusivity afforded to pediatric drugs can present commercial obstacles.
In summary, the pediatric drugs market, while niche, plays an indispensable role within the pharmaceutical industry by addressing the distinctive healthcare needs of the pediatric population. The market's expansion is spurred by the rising prevalence of pediatric health conditions, growing awareness, and governmental backing.
Nonetheless, challenges related to clinical trials and pharmaceutical formulation persist, while opportunities arise from collaborative research, research advancements, and innovative drug delivery solutions. Ultimately, the pediatric drugs market serves as a vital guardian of the health and well-being of the youngest members of society.
| Report Coverage | Details |
| Market Size by 2034 | USD 460.12 Billion |
| Market Size in 2024 | USD 145.53 Billion |
| Market Growth Rate from 2024 to 2034 | CAGR of 12.20% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2034 |
| Segments Covered | Type, Route of Administration, Distribution Channel, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Rising pediatric health issues
The expansion of the pediatric drugs market is notably underpinned by the surging incidence of pediatric health challenges. The increasing occurrence of childhood health issues, encompassing conditions like childhood obesity, diabetes, asthma, and diverse neurological disorders, has engendered a compelling demand for specialized pharmaceuticals designed exclusively for the pediatric demographic. Children represent a unique patient population with distinct physiological and developmental characteristics. These distinctive health challenges necessitate the development of tailor-made drug formulations that cater to their specific needs effectively.
As a result, the pharmaceutical industry is placing greater emphasis on research and the creation of drugs uniquely tailored for pediatric patients. The escalating demand for pediatric medications is further intensified by the growing awareness among parents, caregivers, and healthcare practitioners about the necessity of addressing the particular healthcare requirements of children. This advocacy for pediatric drug development has gained traction, prompting governments and regulatory bodies to introduce incentives and programs aimed at fostering research in this domain.
These initiatives, such as market exclusivity and extended patent protection for pediatric drugs, have attracted pharmaceutical investment and spurred innovation. In sum, the upsurge in pediatric health issues is driving the growth of the pediatric drug market, solidifying its pivotal role in safeguarding and enhancing the well-being of children worldwide.
Limited market exclusivity
The limited duration of market exclusivity stands out as a substantial impediment to the expansion of the pediatric drugs market. In contrast to drugs created for adults, pediatric medications often enjoy shorter periods of market exclusivity and patent protection. These constraints diminish the financial incentives available to pharmaceutical companies for investing in pediatric drug development. Pharmaceutical enterprises are typically drawn to markets where they can recoup their investments in research and development and yield substantial profits.
However, the smaller patient populations and briefer exclusivity periods in the pediatric sector often make it more challenging to justify resource allocation for research and development efforts. This limitation can notably impact the development of drugs for rare pediatric diseases, where patient numbers are exceptionally limited.
As a consequence, many children continue to receive off-label use of medications intended for adults, potentially posing risks to safety and effectiveness. Addressing this constraint necessitates policy and regulatory reforms that provide stronger incentives for pediatric drug development, ensuring that children have access to secure and efficacious treatments.
Gene therapy advancements
Age-appropriate formulations represent a significant opportunity in the pediatric drugs market. The unique healthcare needs of children require medications that are not only safe and effective but also palatable and easy for them to take. Developing innovative age-appropriate formulations has the potential to address these specific needs and improve patient compliance and treatment outcomes. Opportunities arise in several ways. Pharmaceutical companies and formulation experts are focusing on creating medications in various dosage forms that are appealing to children, such as chewable tablets, oral liquids with pleasant flavors, and dissolvable formulations. Taste masking techniques and user-friendly packaging play a crucial role in making medications more acceptable to young patients.
Moreover, age-appropriate formulations extend beyond mere palatability. They include precise dosing systems, ensuring that children receive the right amount of medication. This can help reduce dosing errors and improve treatment efficacy.
In summary, the development of age-appropriate formulations is not only an ethical imperative but also a viable business opportunity. It meets the distinctive requirements of pediatric patients, enhancing their adherence to treatment and overall health outcomes. This focus on pediatric formulation innovation fosters growth and advancement within the pediatric drugs market.
The respiratory disorder drugs segment has held a 35% revenue share in 2023. The dominance of the respiratory disorder drugs segment within the market can be attributed to several compelling factors. Firstly, the global prevalence of respiratory ailments, encompassing conditions like chronic obstructive pulmonary disease (COPD), asthma, and respiratory infections, propels the demand for medications tailored to address these health challenges.
Secondly, the growing consciousness surrounding respiratory health, accentuated by environmental influences such as air quality and the recent COVID-19 pandemic, has amplified the necessity for efficacious drugs targeting respiratory issues.
Furthermore, the continual advancements and research endeavors in this segment, spanning from biologics to inhalation therapies, consistently expand the growth prospects of this market. In combination, these elements firmly establish the respiratory disorder drugs segment as a pivotal force within the pharmaceutical landscape.
The gastrointestinal drugs segment is anticipated to expand at a significant CAGR of 13.7% during the projected period. The dominance of the Gastrointestinal Drugs segment in the pediatric drugs market is primarily attributed to the high incidence of gastrointestinal ailments among children. These disorders, ranging from reflux and irritable bowel syndrome to inflammatory bowel diseases, demand specialized treatments tailored for pediatric patients.
Additionally, infants and children frequently grapple with gastrointestinal problems like gastroenteritis, creating a critical need for pediatric gastrointestinal medications. Pharmaceutical firms are proactively engaged in crafting child-friendly formulations, encompassing liquids and chewable tablets. The surging requirement for safe and efficacious remedies for these prevalent pediatric conditions, bolstered by regulatory backing and growing awareness, firmly establishes the gastrointestinal drugs segment as a prominent contributor to the pediatric drugs market.
The oral segment had the highest market share of 42% in 2023. The oral segment commands a significant share in the pediatric drugs market due to several key factors. Firstly, oral medications are generally more accepted and convenient for pediatric patients, particularly for those who may have difficulty with injections or other forms of drug administration.
Secondly, the availability of pediatric-friendly oral formulations, such as syrups, chewable tablets, and pleasant-tasting liquids, enhances patient compliance. Additionally, oral drugs are often preferred by healthcare providers and caregivers, offering ease of administration and precise dosing, making them a primary choice for pediatric medication, which contributes to their dominant market share.
The parenteral segment is anticipated to expand at the fastest rate over the projected period. The parenteral segment commands a significant growth in the pediatric drugs market due to several factors. Parenteral medications, administered through injections, intravenous infusions, or other non-oral routes, offer precise dosing and immediate drug delivery, critical in pediatric cases where accuracy is paramount. In emergencies, these routes are often preferred.
Additionally, they bypass issues related to oral medication palatability and gastrointestinal absorption, common challenges in pediatrics. The Parenteral segment also caters to the delivery of critical care medications, vaccines, and treatments for severe illnesses, making it a cornerstone of the pediatric drugs market.
The hospital pharmacies segment had the highest market share of 46% in 2023. The hospital pharmacies segment commands a significant share in the pediatric drugs market due to several key factors. Hospitals are primary healthcare centers for children, making them crucial points of access for pediatric medications. Hospital pharmacies are equipped to manage and dispense specialized pediatric formulations, ensuring accurate dosing and safety.
Additionally, these pharmacies play a pivotal role in providing medications for critically ill pediatric patients. Collaborations between pharmaceutical companies and hospitals facilitate clinical trials and research, further strengthening the influence of hospital pharmacies in the pediatric drug market. Overall, their expertise, infrastructure, and accessibility make hospital pharmacies dominant players in the sector.
The online pharmacies segment is anticipated to expand at the fastest rate over the projected period. The online pharmacies segment commands significant growth in the pediatric drugs market due to its convenience and accessibility. Online pharmacies offer a wide range of pediatric medications, enabling parents and caregivers to access treatments for children from the comfort of their homes. This is particularly crucial for pediatric medications, as caregivers often prefer convenient solutions.
Additionally, online platforms provide information and resources, aiding informed decision-making. The COVID-19 pandemic further accelerated the adoption of online pharmaceutical services, reinforcing the dominance growth of this segment in the pediatric drugs market as a preferred and accessible distribution channel.
Segments Covered in the Report
By Type
By Route of Administration
By Distribution Channel
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
February 2025
February 2025
August 2024
January 2025