April 2025
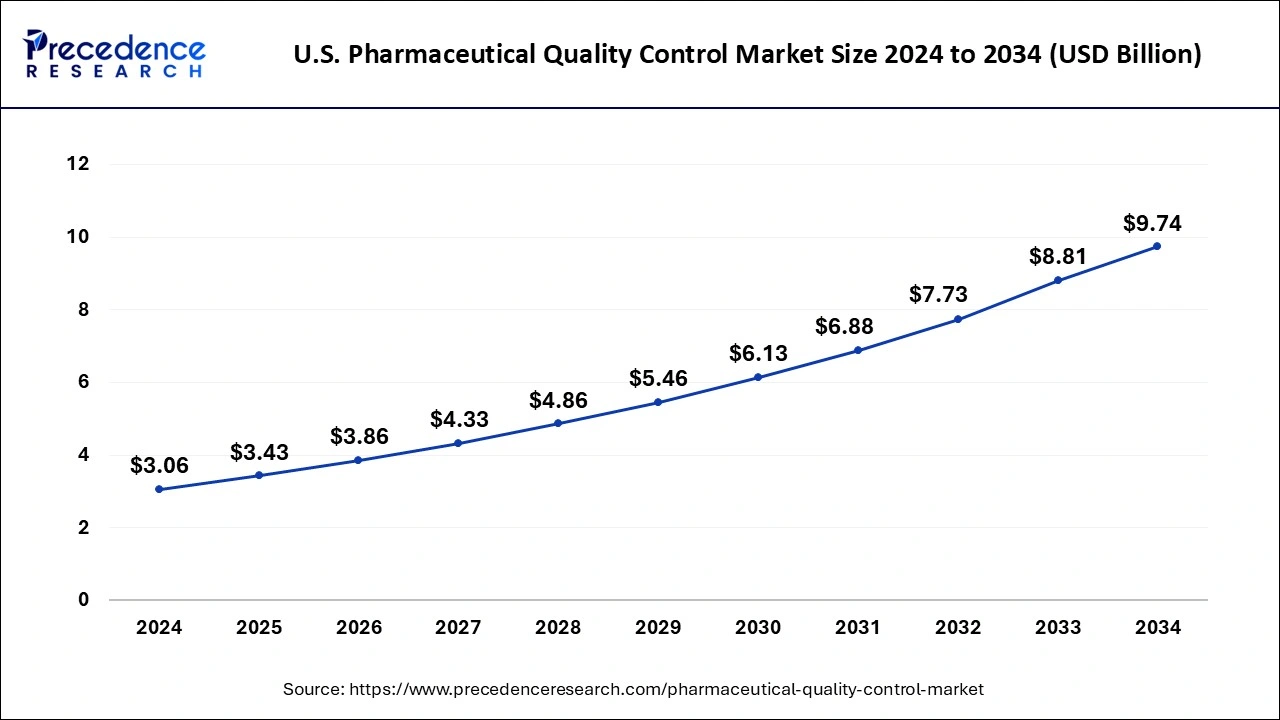
The global pharmaceutical quality control market size is calculated at USD 14.43 billion in 2025 and is forecasted to reach around USD 40.16 billion by 2034, accelerating at a CAGR of 12.06% from 2025 to 2034. The North America market size surpassed USD 4.37 billion in 2024 and is expanding at a CAGR of 12.10% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global pharmaceutical quality control market size was calculated at USD 12.86 billion in 2024 and is predicted to increase from USD 14.43 billion in 2025 to approximately USD 40.16 billion by 2034, expanding at a CAGR of 12.06% from 2025 to 2034. The rising demand for medication due to the rising prevalence of chronic diseases is driving the growth of the market.
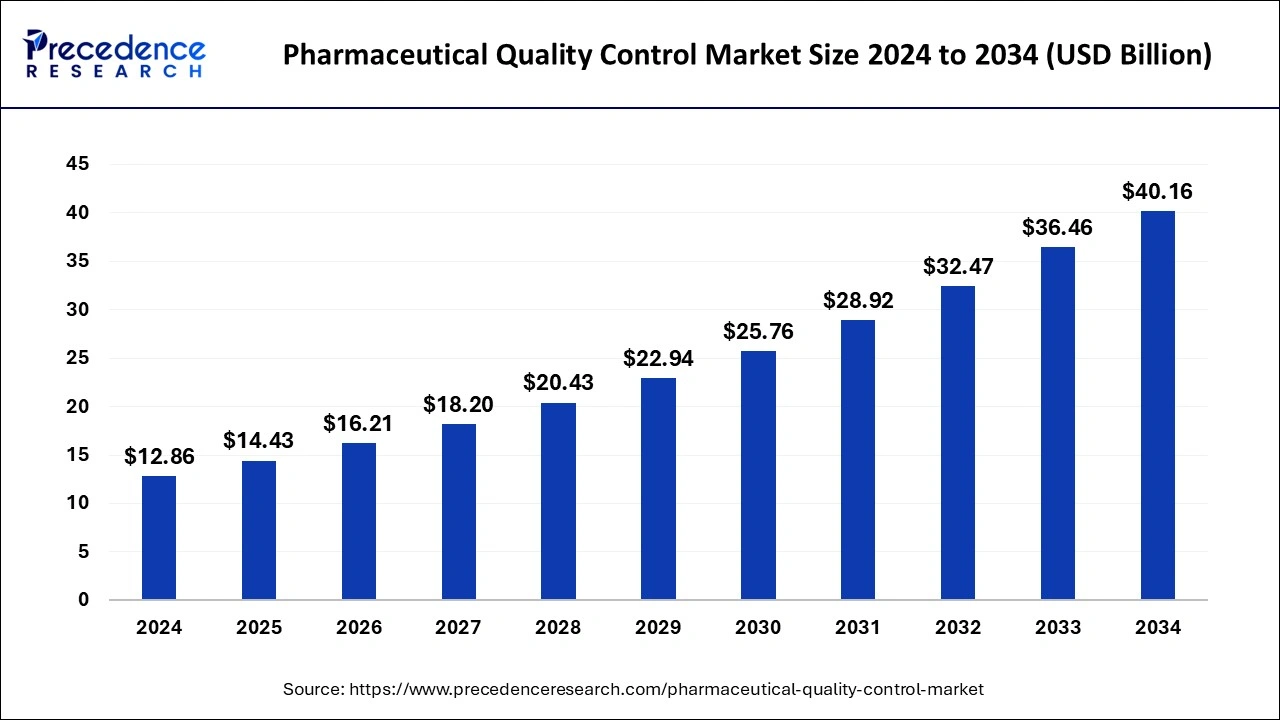
The U.S. pharmaceutical quality control market size was exhibited at USD 3.06 billion in 2024 and is projected to be worth around USD 9.74 billion by 2034, growing at a CAGR of 12.28% from 2025 to 2034.
North America led the pharmaceutical quality control market with the largest market share in 2024. The growth of the market in the region is increasing due to the well-developed healthcare infrastructure and the rising investment by pharmaceuticals in drug development and launch that drives the growth of the market in the region. The growth in the pharmaceutical and healthcare industry are driving the demand for the quality control unites for the detection and safety of the medical drugs. The higher availability of the major healthcare centers and pharmaceutical giants are accelerating the growth of the pharmaceutical quality control market in the region.
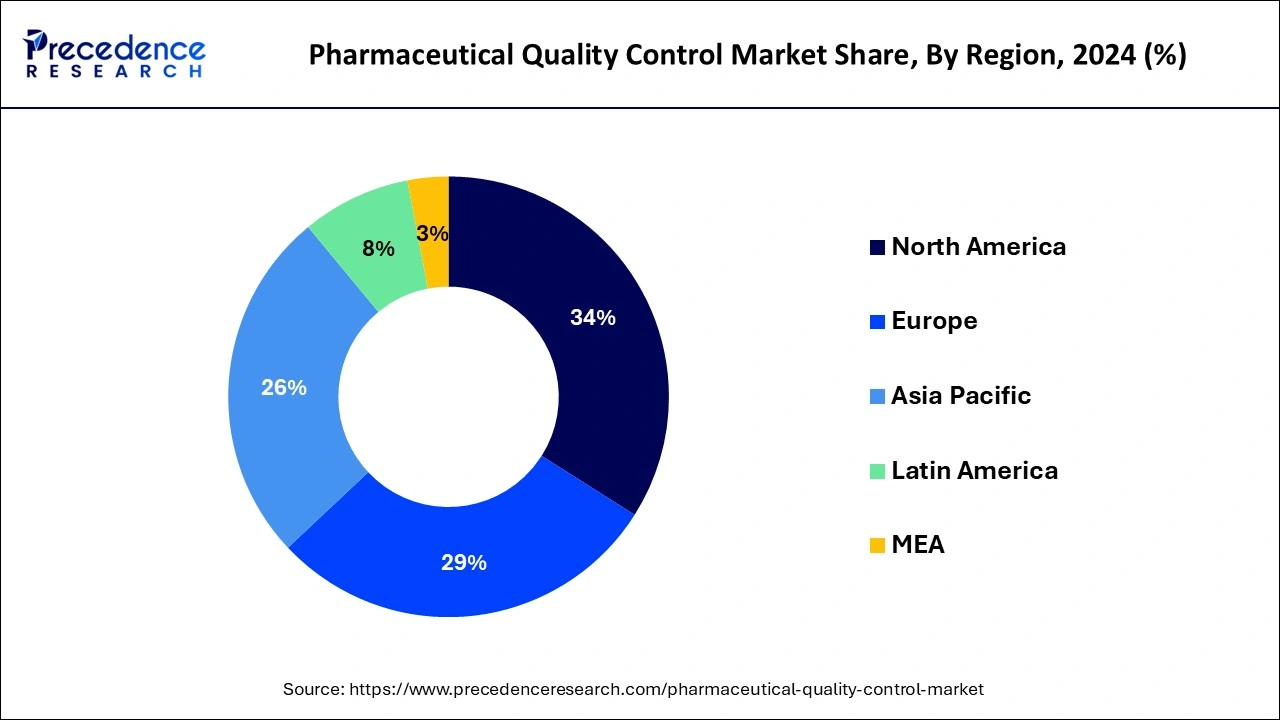
Asia Pacific is expected to witness the fastest growth in the pharmaceutical quality control market during the forecast period. The growth of the market in the region is expected to rise due to the rising population and the growing demand for the effective and safer drug for the treatment of the diseases. The rising interventions of the regional governments for the development of healthcare infrastructure, and pharmaceutical industry are driving the demand for the quality control units in the pharma industry that contributed to the expansion of the pharmaceutical quality control market in the region.
The pharmaceutical quality control market offers services that are responsible for ensuring the purity and identity of a particular pharmaceutical. Quality control ensures the quality of the drug once it is fully manufactured, it tests and approves the drug quality as per the set regularity standards. Quality control departments in the pharmaceutical industry are important to the efficiency, safety, and consistency of pharmaceutical products. It works on the inspection and safety of the drug for detecting and preventing defects in the manufacturing process. The rising demand for healthcare infrastructure and medication along with the investments in research and development activities in the pharmaceutical industry is driving the growth of the pharmaceutical quality control market.
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 12.06% |
| Market Size in 2025 | USD 14.43 Billion |
| Market Size in 2024 | USD 12.86 Billion |
| Market Size by 2034 | USD 40.16 Billion |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product, Analysis Type, and Product Tested |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Increasing chronic diseases
The rise in the prevalence of the chronic diseases is driving the demand for the effective and safer drug and treatment medication which drives the development in the pharmaceutical industry and further contributes for the growth of the pharmaceutical quality control market. The rising aging population and the environmental conditions are the factors that cause severe illness.
Additionally, the rising shift in the lifestyle and the adoption of smoking and increased consumption of alcohol by the younger population are results in the increasing chronic diseases such as cancer, cardiovascular diseases, respiratory illness, etc. that drives the demand for the effective treatment medication that driving the demand for the innovation in the drug development that directly contributed to the expansion of the pharmaceutical quality control market.
Lack of professionals
Quality control in the pharmaceutical industry often requires specialized techniques and instruments for analyzing raw materials, intermediates, and finished products. These techniques, such as chromatography, spectroscopy, and mass spectrometry, require skilled professionals with in-depth knowledge and experience to operate effectively and interpret results accurately. Quality control processes are critical for identifying potential issues such as impurities, contaminants, or deviations from specifications that could affect the safety, efficacy, or stability of pharmaceutical products. Without skilled professionals to perform thorough and accurate analyses, there's an increased risk of errors or oversight, which could lead to product recalls, regulatory sanctions, or compromised patient safety. Thereby, the factor is observed to restrain the growth of the pharmaceutical quality control market.
Technological advancements
The increasing adoption of technological advancements in the quality control in pharmaceutical is observed to offer lucrative opportunities for the pharmaceutical quality control market’s growth. The continuing advancements of technologies in the healthcare sector are enhancing the quality and safety of the drug and driving the growth of the market. The rising demand for the healthcare infrastructure and the rising investment by the government for the development and the launch of pharmaceutical units are results in the increased demand for the pharmaceutical quality control for detecting and preventing any defects in the drug manufacturing process. Thus, the rising demand for pharmaceuticals industry and technological advancements are positively influencing the growth of the market.
The services segment dominated the market with the highest share in 2024. The growth of the segment is attributed to the rising demand for quality control services in the pharmaceutical industry for maintaining the safety of the drug and detecting manufacturing defects. Additionally, the rising investment in the research and development activities in the development of the novel drugs for that drives the expansion of the pharmaceutical quality control services.
The consumables segment is expected to grow at a significant rate during the forecast period. The growth of the segment is expected to dominate due to the rising demand for the medical consumables such as bandages, syringes, gloves, cotton wool, catheters, infusion sets, sutures, and intravenous cannulas, etc. the rise in the surgical procedures and maintaining the hygiene and safety from the bacterial infections that are driving the demand for the pharmaceutical consumables.
The sterile testing segment dominated the pharmaceutical quality control market in 2024. The rising demand for the sterile testing of pharmaceutical products for removing viable microorganisms. Sterile testing also depends on the procedural methods which can actively prevent contamination of biological products, like goods manufacturing process, and clean room technology. WHO assigns the sterile testing that are applicable for the various range of the biological medicinal products like blood products, vaccines, biotechnology products, cell and tissue products, etc. the increasing demand for the sterile testing in the pharmaceutical industry for ensuring the drug safety.
The raw material testing segment is showing an exponential growth in the market during the upcoming period. Raw material testing is one of the important parts of pharmaceutical products manufacturing. It is crucially important to test each raw material before manufacturing drug and food products. As per the FDA guidelines it is mandatory to check the quality of the raw material for manufacturing of the drug and food products. Quality checking of the raw material before starting the manufacturing process is highly important. It reduces the chances of manufacturing low-quality finished products and reduces the product recall possibilities.
The vaccine segment dominated the pharmaceutical quality control market in 2023. The higher demand for the quality control application in the vaccine due to the higher use of the vaccine in the treatment of several health issues. The increasing demand for several vaccinations from the prevention of infectious diseases and other chronic illnesses is again observed to expand the segment’s growth. Vaccines are more effective in treatment and reduces the recovery time of the patients, though the drug is directly injected to the blood stream in the body. The increasing demand for the vaccines in the future for fighting against the diseases like Covid-19 and others are driving the demand for the vaccine testing for ensuring the safety and manufacturing defects.
By Product
By Analysis Type
By Product Tested
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
April 2025
April 2025
February 2025
September 2024