August 2024
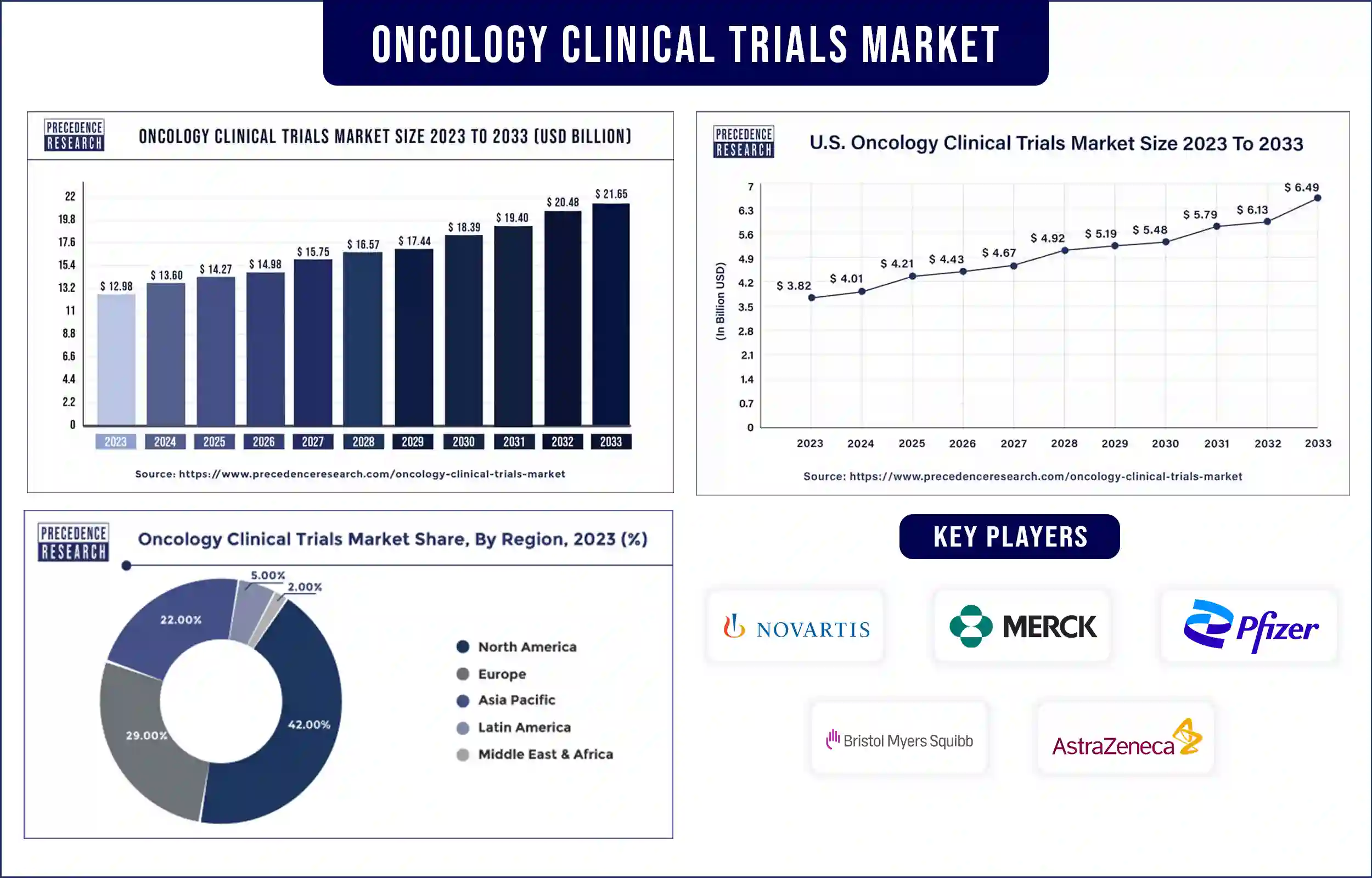
The global oncology clinical trials market revenue was valued at USD 12.98 billion in 2023 and is poised to grow from USD 13.60 billion in 2024 to USD 21.65 billion by 2033, at a CAGR of 5.30% during the forecast period 2024 – 2033. The growth of the oncology clinical trials market is mainly driven by the increasing number of clinical trials, expanding the investment in pharmaceutical R&D, and increasing medications in the pipeline.
Oncology clinical trials are the trials accomplished under clinical research studies and follow a controlled procedure. These trials are primarily accomplished to obtain the data connected with the security and efficacy of modern advanced drugs. Clinical trial information is necessary for drug consent and for it to be supplied in the market. These experiments are performed under different stages, which are subjected to many factors.
The growing number of individuals diagnosed with cancer is expected to rise multi-fold in the forthcoming years. Even though patients have amplified suggestively due to research studies in the oncology sector, the number of cancer patients is projected to rise globally. Among all, lung cancer is considered the top reason for death because of cancer death, with a probability of 1.9 million people detected yearly across the world. It is considered that the Phase II segment liberated the oncology clinical trials market because of the growing number of researchers in Phase II.
The trend of digital transformation in the field of oncology clinical trials is boosting the market. This oncology clinical trials market is rapidly adopting advanced digital technologies to modernize operations, improve customer practice, and enhance efficacy. The incorporation of digital technologies such as wearable gadgets, telemedicine platforms, and mobile applications is transforming oncology clinical trials.
Artificial intelligence and automation technologies are changing markets extending from manufacturing to healthcare services. Startups that develop AI-driven solutions, like robotic process automation or machine learning algorithms, have important development capacity. These facilitate remote observation of patients and enhance patient engagement and real-time information gathering which leads to more effective and patient-centric trials.
Suggesting treatments according to a patient’s genetic makeup and tumor appearance is gradually becoming more common. This trend is enhancing growth in biomarker-driven clinical trials, where particular genetic biomarkers or mutations are focused on with special personalized treatment.
There is a drastic transfer towards immunotherapy in oncology clinical trials. With its effective results in several cancers such as lung cancer, melanoma, and many more, several trials are concentrating on immunotherapeutic methods such as CAR-T cell therapy and checkpoint inhibitors.
Increasing cancer patients and drug companies driving the oncological trial market
There has been an increasing number of cases of cancer fighters staying in the U.S. Numerous biopharmaceutical study centers are emerging with effective and better-tolerated therapies to meet the requirements of patients. Growing association among many market companies has directed market progress. This oncology clinical trials market information gives details about new current developments, trade guidelines, import-export study, production study, value chain optimization, market stake, the influence of domestic and confined market companies, analyses chances in terms of evolving income pockets, variations in market guidelines, planned market growth study, market extent, category market developments, application positions and supremacy, product consents, product promotions, geographic extensions, technological inventions in the market.
On the other hand, experiments in oncology are emerging and evolving quickly. Therefore, not many people, but some experts, are well aware of the actual procedure of the experiment. These processes need extremely high competence as they are very delicate procedures, and a minor error can cause many difficulties. Therefore, this feature leads to market interruption.
| Report Attribute | Key Statistics |
| Market Revenue in 2024 | USD 13.60 Billion |
| Market Revenue by 2033 | USD 21.65 Billion |
| Market CAGR | 5.30% from 2024 to 2033 |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Recent Development by Labcrop
North America dominated the oncology clinical trials market worldwide in 2023. Moreover, the growing adoption of advanced technologies in clinical research studies and government funding will additionally improve the market development in the area during the predicted period. The U.S. dominantly contributes to the growth of the market due to advanced healthcare infrastructure, the presence of major market players, and continuous research and development.
Apart from this, the U.S. is among the top countries with major cancer cases and cancer-related deaths, which demand better treatment options. Due to the presence of major market players and government laboratories, the country continuously conducts clinical trials in order to develop vaccines, medicines, and treatments for cancer. The FDA plays a major role in the approval of different phases of clinical trials and final approval before the launch of the product/treatment in the market for public use.
The European oncology clinical trials market is poised for robust growth, driven by ongoing advancements in cancer research, supportive regulatory environments, and increasing investments. The focus on personalized medicine and innovative therapies will likely continue to shape the market dynamics, leading to improved cancer treatment outcomes. In summary, the European oncology clinical trials market presents significant growth opportunities, driven by the rising incidence of cancer, advancements in medical research, and favorable regulatory frameworks. Key players are expected to continue their investment in innovative treatments, ensuring the market's dynamic and evolving nature.
The market is set for continued growth, driven by advancements in cancer research, supportive regulations, and increased healthcare investments. Focus on personalized medicine and innovative therapies will shape future trends. In summary, the Asia Pacific oncology clinical trials market is poised for robust growth, supported by rising cancer incidence, technological advancements, and regulatory support, offering significant opportunities for key players.
Increasing demand for experiments enhancing the oncology clinical trials market
There is a growing number of research in phase II, which results in market expansion. Besides, for several years, there has been an enormous development in the total efficiency of oncology clinical trials, estimated as accomplishment rates compared to the experiment effort by 25%. Furthermore, the Medicines Healthcare Products Regulatory Agency (MHRA), within a span of a few years, increased the research applications for Phase II and Phase III trials by 5.7%. Therefore, this factor enhances the speedy growth of the market. The digital alteration wave offers an assembly of avenues for the oncology clinical trials market to take advantage of. With the circulation of knowledge and the net, corporations can hold digital stages for advertising, sales, and customer appointments, thereby growing their reach worldwide while dropping operative costs.
The growing focus on sustainability and ecological awareness opens up noteworthy chances for the oncology clinical trials market to accept eco-friendly performances and develop supportable products and service areas. As customers become more ecologically conscious, there is a rising request for properly tracked goods, renewable energy resolutions, and eco-friendly replacements across numerous businesses. Corporations that arrange sustainability not only donate to ecological preservation but also receive a competitive authority by alluring to the morals of careful customers.
Market Segmentation
By Phase Type
By Study Design
Buy this Research Report@ https://www.precedenceresearch.com/checkout/3672
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308
August 2024
January 2025
January 2025
January 2025