March 2025
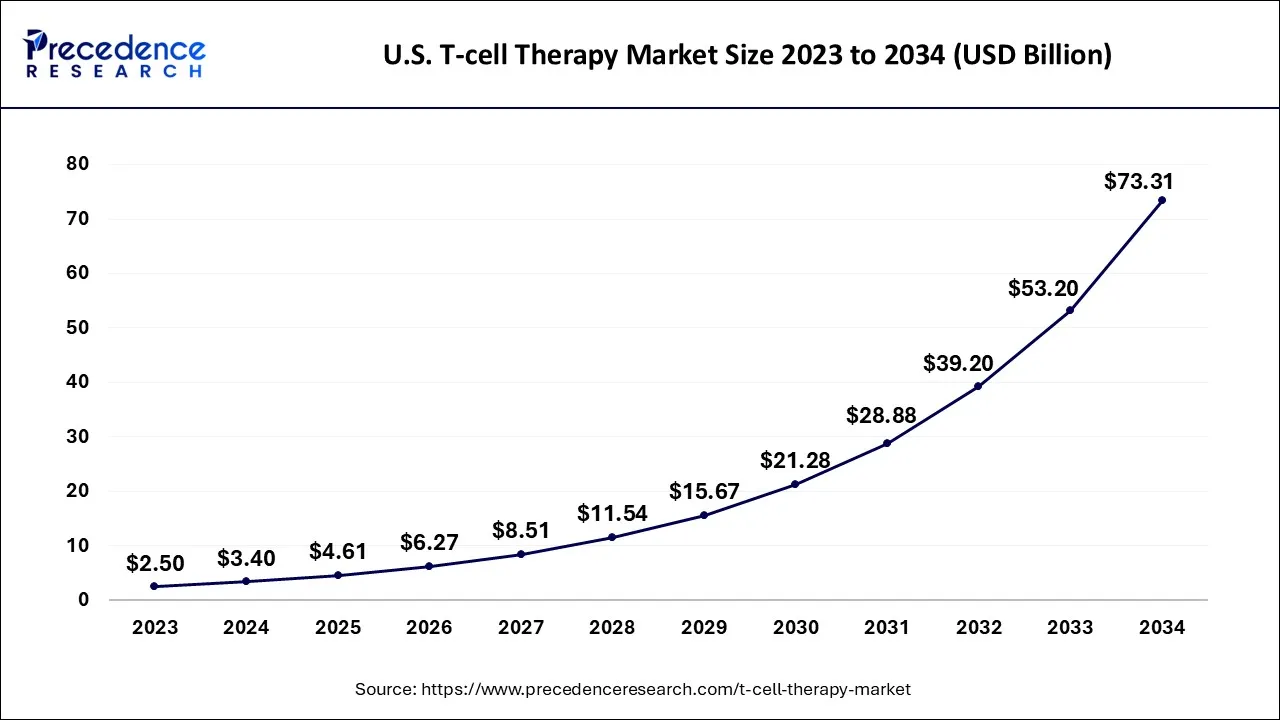
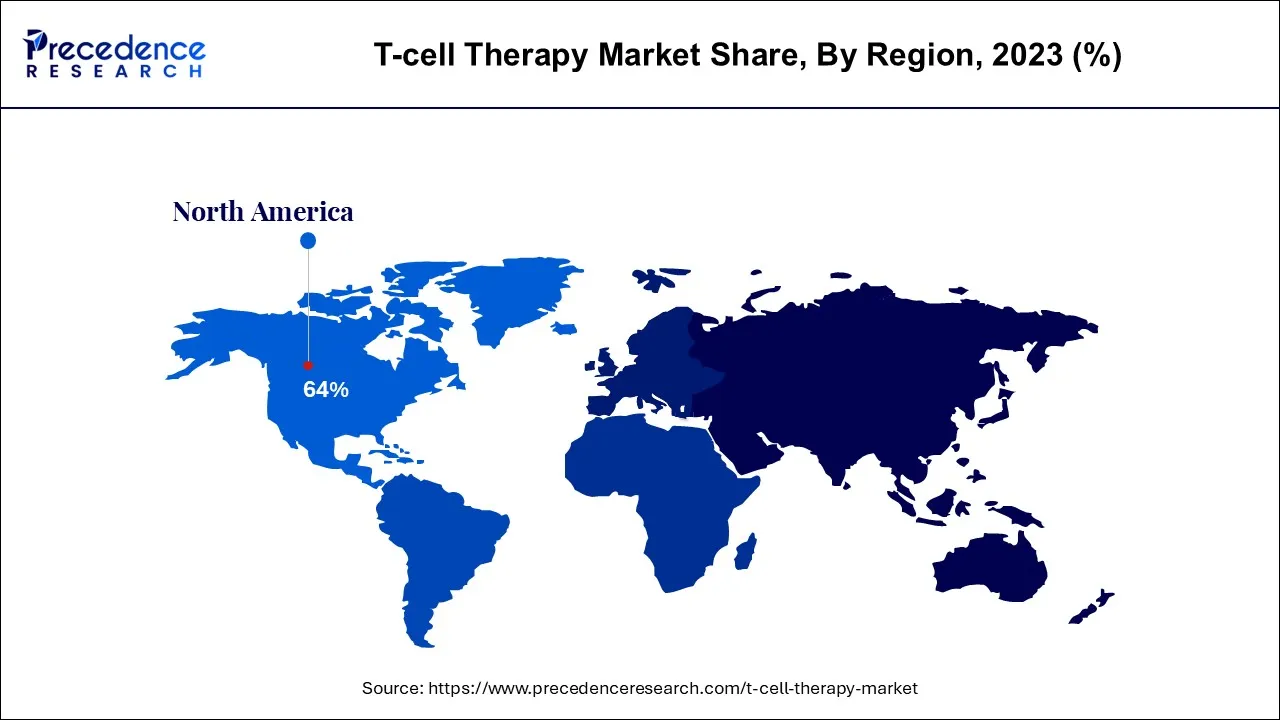
The global T-cell therapy market size is calculated at USD 7.59 billion in 2024, grew to USD 10.30 billion in 2025, and is predicted to hit around USD 161.21 billion by 2034, poised to grow at a CAGR of 35.74% between 2024 and 2034. The North America T-cell therapy market size accounted for USD 4.86 billion in 2024 and is anticipated to grow at the fastest CAGR of 35.85% during the forecast year.
The global T-cell therapy market is expected to be valued at USD 7.59 billion in 2024 and is anticipated to reach around USD 161.21 billion by 2034, expanding at a CAGR of 35.74% over the forecast period from 2024 to 2034.
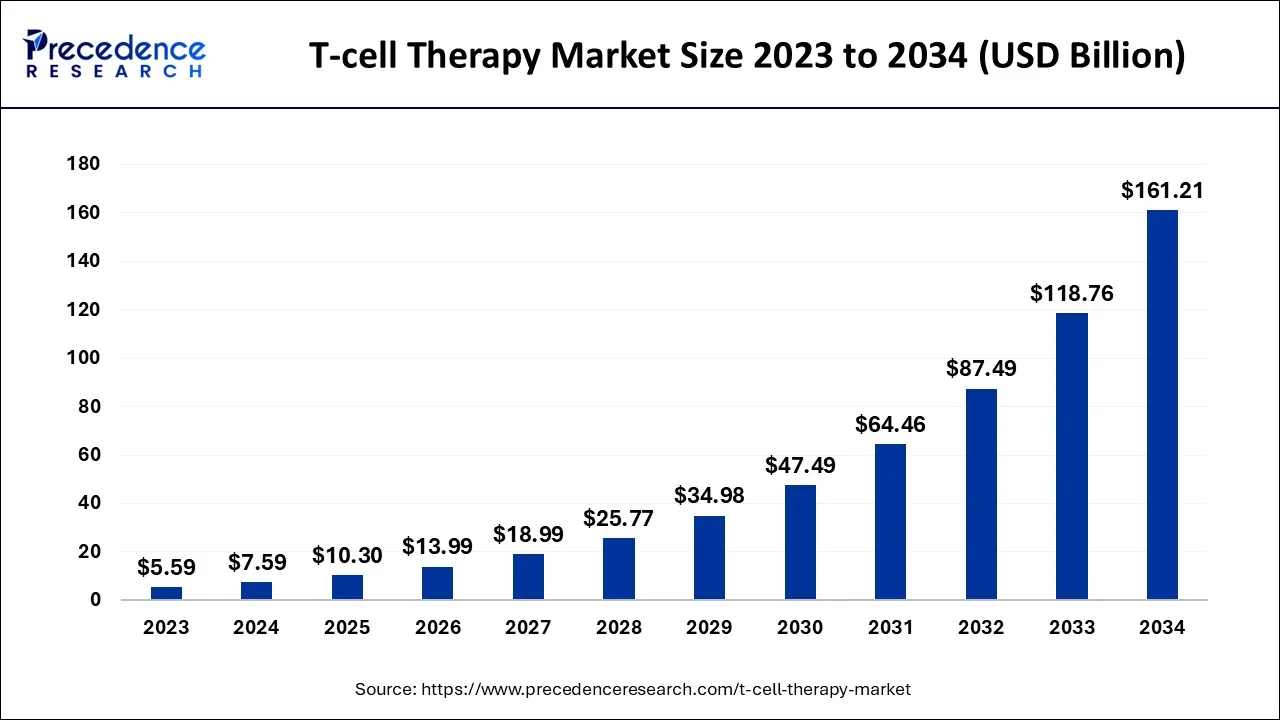
The U.S. T-cell therapy market size is accounted for USD 3.40 billion in 2024 and is projected to be worth around USD 73.31 billion by 2034, poised to grow at a CAGR of 35.94% from 2024 to 2034.
North America is expected to dominate the market over the forecast period. The market growth in the region is owing to the increasing clinical trials and approvals. North America, particularly the United States, has been at the forefront of T-cell therapy development. Several clinical trials investigating the safety and efficacy of CAR T cell therapies have been conducted in the region. In addition, The U.S. Food and Drug Administration (FDA) has granted approvals to CAR T cell therapies for certain indications.
Asia Pacific is expected to grow at the highest CAGR during the forecast period. The market is expanding in this area due to reasons such as the rising prevalence of autoimmune diseases and cancer, rising healthcare expenditures, and growing public awareness of the benefits of T-cell therapy. China, Japan, and India are the three countries with the greatest market shares in the Asia-Pacific T-cell therapy market. It is projected that these countries' expanding healthcare infrastructure investments and huge patient populations would drive market growth.
Europe is expected to hold a substantial market share over the forecast period. The region has been actively involved in T-cell therapy research and clinical trials. Academic institutions, research centers, and hospitals in countries like Germany, the United Kingdom, France, and others have contributed to the development and testing of T-cell therapies. Moreover, European countries with advanced healthcare systems and specialized oncology centers are equipped to deliver T-cell therapies. These centers provide the necessary facilities, expertise, and support for patient evaluation, treatment administration, and follow-up care. Thus, this is expected to drive the market growth in the region.
T-cell therapy is a type of medical treatment that harnesses the power of a specific subset of immune cells called T cells to target and destroy diseased cells, particularly cancer cells. T cells are a crucial component of the immune system, responsible for recognizing and eliminating abnormal cells, including those infected by viruses or transformed into cancerous forms. T-cell therapy involves modifying or enhancing a patient's T cells to make them more effective in targeting and eliminating specific disease-causing cells. The rising cases of cancer across the globe are observed to supplement the growth of the market. As per the statistics by IARC, it is anticipated that there are going to be approximately 28 million new cancer cases worldwide each year by 2040 if incidence remains steady and population growth and aging continue at current rates.
The T-cell therapy market is being driven by numerous factors including the growing prevalence of cancer, rising FDA approvals, growing collaboration among the market players, and rising government initiatives. Moreover, the healthcare sector is focused on offering multiple immunotherapies to patients. This factor also promotes the growth of the market.
| Report Coverage | Details |
| Market Size in 2024 | USD 7.59 Billion |
| Market Size by 2034 | USD 161.21 Billion |
| Growth Rate from 2024 to 2034 | CAGR of 35.74% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2034 |
| Segments Covered | By Therapy Type, By Indication, and By End User |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Increasing prevalence of cancer
The global burden of cancer continues to rise, with a growing number of patients diagnosed each year. T-cell therapy presents a novel approach that addresses unmet medical needs, providing an alternative for patients who have exhausted conventional treatment options. For instance, according to the National Center for Health Statistics, the United States is expected to witness 1,958,310 new cancer cases and 609,820 cancer-related deaths in 2023. Therefore, the increasing prevalence of cancer is expected to propel the T-cell therapy market during the forecast period.
High cost
T-cell therapy, particularly CAR T-cell therapy, can be expensive to develop, manufacture, and administer. The complex process of modifying a patient's T cells, the need for specialized facilities, and the personalized nature of the treatment contribute to high costs. These costs can limit accessibility for patients and strain healthcare systems. For instance, according to the Journals of the American Medical Association, the cost of CAR T-cell therapy is around USD 373,000 to USD 475,000. Thus, the high cost of therapy limits patients and acts as a major restraint for the market.
Increasing approvals for therapies
The increasing approvals by the Food and Drug Administration (FDA) and other agencies in the area of cancer cell therapy are expected to provide a potential opportunity for market growth over the forecast period. For instance, in May 2022, Penn Medicine developed CAR T cell therapy and won FDA's third approval. The FDA and other agencies for approval of therapies have noticed the importance of cell therapies, especially while considering the rising number of cancer patients. Moreover, strict and firm regulatory framework for the development and trials of these therapies provided by FDA is being followed by developers and researchers. Thus, it becomes easier to approve therapies. Hence, the increasing rate of approval for therapies by FDA highlights the market’s development.
Based on the therapy type, the global T-cell therapy market is segmented into CAR T-cell therapy, T Cell Receptor (TCR)-based and Tumor Infiltrating Lymphocytes (TIL)-based. The CAR T-cell therapy is expected to dominate the market over the forecast period. CAR T cell therapy has shown impressive response rates, with a substantial number of patients achieving complete remission in tumor burden. This is particularly notable for patients who have not responded well to other treatments.
Moreover, in some cases, CAR T cell therapy has resulted in long-lasting remissions, even in patients with advanced or refractory cancers. This can significantly improve the quality of life and prognosis for these patients. Furthermore, CAR T cell therapy is personalized to each patient's unique immune system and disease characteristics. The patient's T cells are modified and engineered to target specific cancer antigens, making the treatment more targeted and potentially more effective. Thus, owing to these benefits CAR T-cell therapy is expected to dominate the market during the forecast period.
Based on the indication, the global T-cell therapy market is segmented into hematologic malignancies, solid tumors, and others. The hematologic malignancies segment is expected to capture a significant market share over the forecast period and it is further sub-categorized into lymphoma, leukemia, and myeloma.
The leukemia sub-segment is expected to dominate the market over the analysis period. T-cell therapy, particularly CAR T cell therapy, has made significant strides in the treatment of leukemia, primarily in pediatric and young adult patients with relapsed or refractory acute lymphoblastic leukemia (ALL).
Based on the end user, the global T-cell therapy market is segmented into hospitals and cancer treatment centers. The hospital segment is expected to grow at a significant rate over the forecast period. Hospitals with advanced oncology departments and specialized centers for cellular therapies are equipped to provide T-cell therapy. These institutions have the necessary laboratory facilities, skilled personnel, and multidisciplinary teams to ensure safe and effective T-cell therapy administration.
Additionally, hospitals play a crucial role in evaluating patients for T-cell therapy eligibility. A rigorous assessment of a patient's medical history, disease status, and overall health is conducted to determine whether they are suitable candidates for treatment. Thereby, propelling the segment growth over the projected period.
Segments Covered in the Report
By Therapy Type
By Indication
By End User
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
March 2025
February 2025
February 2025
May 2025