December 2024
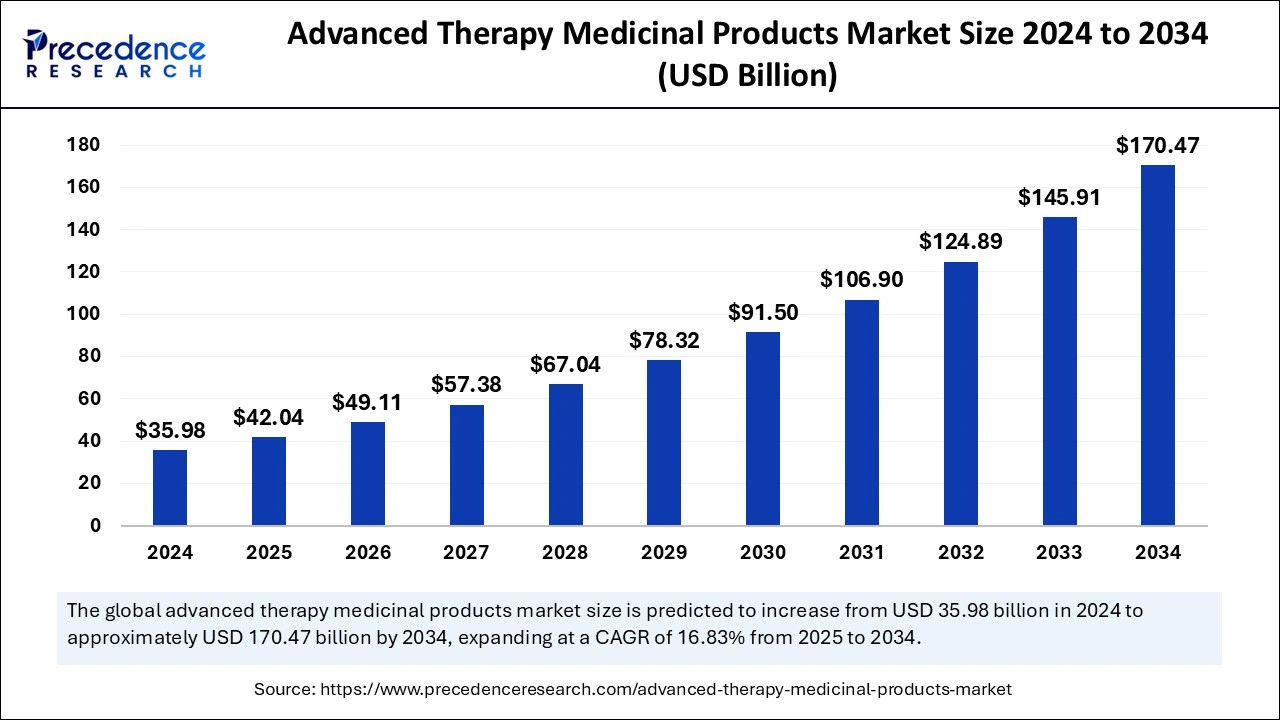
The global advanced therapy medicinal products market size is calculated at USD 42.04 billion in 2025 and is forecasted to reach around USD 170.47 billion by 2034, accelerating at a CAGR of 16.83% from 2025 to 2034. The North America market size surpassed USD 17.63 billion in 2024 and is expanding at a CAGR of 16.92% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global advanced therapy medicinal products market size was estimated at USD 35.98 billion in 2024 and is predicted to increase from USD 42.04 billion in 2025 to approximately USD 170.47 billion by 2034, expanding at a CAGR of 16.83% from 2025 to 2034. The market growth is attributed to the increasing demand for personalized and innovative treatments for chronic and rare diseases.
Artificial Intelligence technologies strengthen all stages of advanced therapy, including the development of medicinal products and manufacturing and regulatory aspects, to boost both efficiency and innovation. By using predictive modeling in the advanced therapy medicinal products market, researchers quickly discover promising gene and cell therapy developments that substantially decrease research expenses at early stages. The application of artificial intelligence within automated manufacturing processes improves ATMP production through optimized cell environment control, reduced variations, and better-quality consistency.
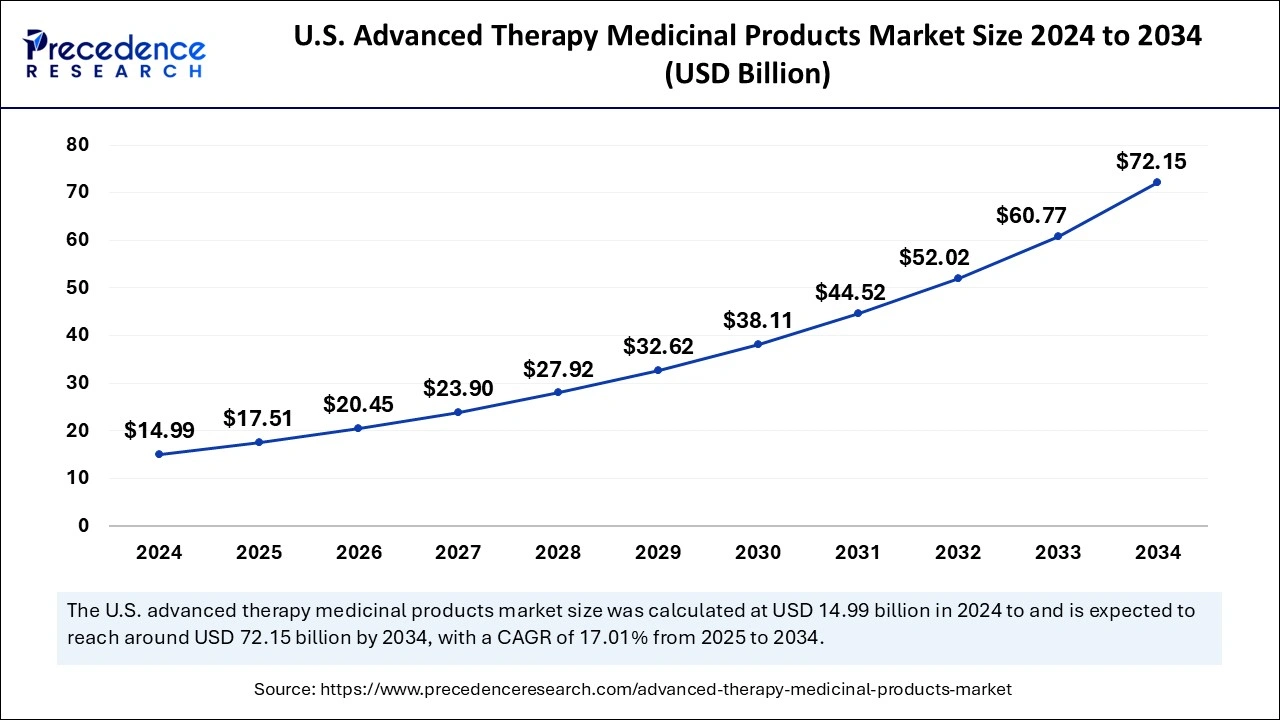
The U.S. advanced therapy medicinal products market size was exhibited at USD 14.99 billion in 2024 and is projected to be worth around USD 72.15 billion by 2034, growing at a CAGR of 17.01% from 2025 to 2034.
North America dominated the global advanced therapy medicinal products market by generating the highest revenue share in 2024 due to regulatory advantages alongside effective product launches and substantial research funding by companies. The U.S. Food and Drug Administration (FDA) allows faster market access through its breakthrough therapy designation program for gene and cell therapies while boosting their market demand. Moreover, the constant gene, cell, and tissue-engineered therapy innovations are supported through regional investments.
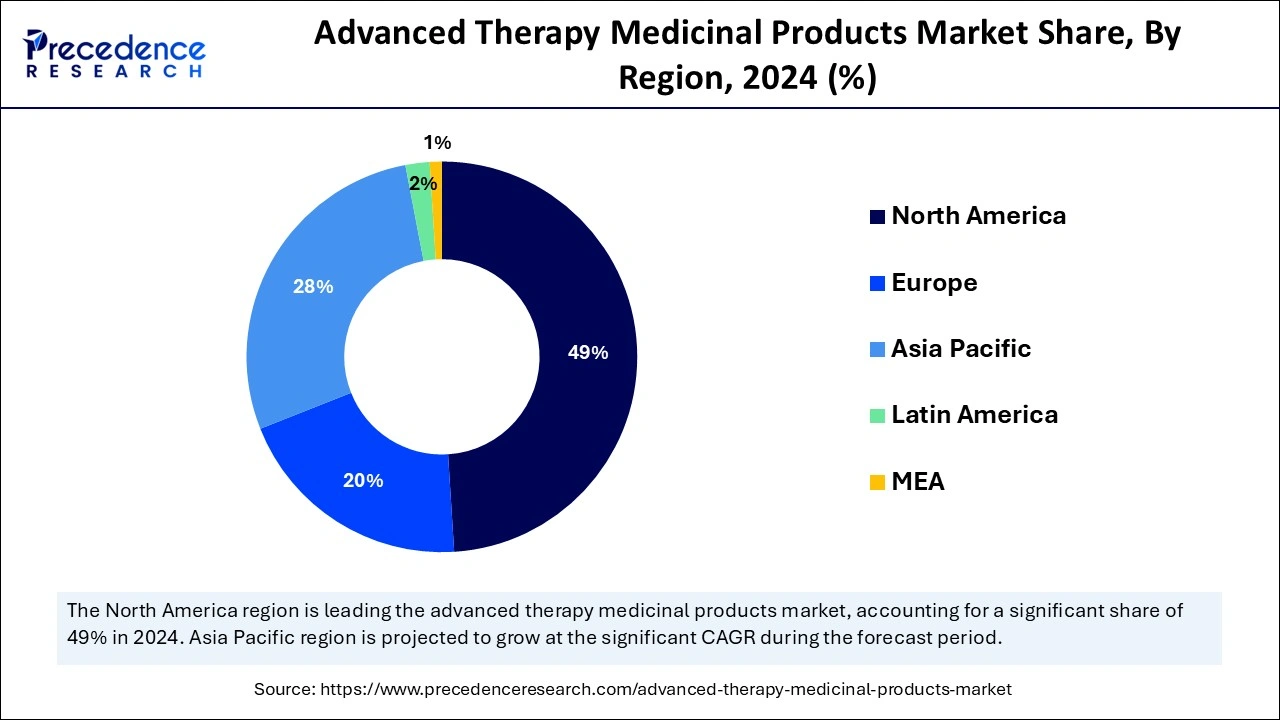
Asia Pacific is projected to host the fastest-growing advanced therapy medicinal products market in the coming years, owing to the growing healthcare demands and rising investments directed toward biotech and regenerative medicine. China's healthcare system enhancements, together with its innovative therapy focus, have fueled the fast expansion of both stem cell-based treatments and gene therapy applications.
Asia Pacific nations actively modernize their approval systems and promote private-public cooperation to advance biotechnology advancement. The Japan Pharmaceuticals and Medical Devices Agency (PMDA) established unique processes for regenerative medicine. Furthermore, the growing governmental subsidies and investment further fuel the advanced therapy medicinal products market in this region.
The advanced therapy medicinal products market continues to grow with increasing disease prevalence. Traditional therapeutic approaches do not offer sustainable treatment solutions to patients suffering from genetic disorders, neurodegenerative diseases, and selected cancers. Advanced therapy medicinal products (ATMPs) that contain gene therapy, cell therapy, and tissue-engineered products address medical needs through their ability to target the fundamental causes of these diseases. Furthermore, the widespread use of ATMPs expands as mounting personalized medicine demands and expanding healthcare accessibility, thus improving treatment prospects for patients who previously had no options.
| Report Coverage | Details |
| Market Size by 2034 | USD 170.47 Billion |
| Market Size in 2025 | USD 42.04 Billion |
| Market Size in 2024 | USD 35.98 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 16.83% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Therapy, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Rising demand for personalized medicine
Rising demand for personalized medicine is anticipated to drive the adoption of advanced therapy medicinal products market as patients and healthcare providers seek targeted treatments with higher efficacy. The growing increase of both chronic conditions and rare diseases enhances the need for advanced therapy medicinal products, as traditional treatment methods prove ineffective in delivering sustained relief.
Among European Union member states, yearly, the population dealing with rare diseases ranges from 27 million to 36 million, while 80% of these cases have genetic roots.
Older adult demographics create additional pressure on regenerative medicine to rebuild cellular operations and enhance lifespan quality. Government entities, together with healthcare institutions, dedicate large budget allocations to research programs that work on developing ATMPs for treating rare and life-threatening diseases. Additionally, the introduction of new ATMPs at biopharmaceutical companies aims to treat medical conditions that have high patient burdens.
High development and manufacturing costs
High development and manufacturing costs are expected to challenge the expansion of the advanced therapy medicinal products market, limiting accessibility for patients. The production process of gene and cell therapies includes demanding bioprocessing techniques in addition to stringent regulatory requirements and special infrastructure facilities that make their manufacturing costs very high. Furthermore, the availability of affordable automated production processes is limited by cost constraints, which makes ATMPs unaffordable in areas where healthcare expenditures are restricted.
High regulatory support for innovative therapies
High regulatory support for innovative therapies is likely to boost the market growth of advanced therapy medicinal products as agencies implement frameworks that streamline ATMP approvals. A faster route to beneficial treatments reached patients through new approval approaches established by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The EMA features a unified ATMP authorization system that executes one issuance evaluation to establish prompt European Union-wide patient pharmaceutical access.
The U.S. Food and Drug Administration demonstrated its urgency regarding important medications for serious conditions by granting priority status to 56% of the 31 drug approvals throughout 2023.
Pharmaceutical companies receive benefits through orphan drug designations, while priority review programs create motivation to develop ATMPs that address rare diseases with no treatment options. The FDA's Office of Orphan Products Development grants medicinal products for rare diseases designation status that provides both tax credits and market exclusivity benefits for pharmaceutical development.
The tissue-engineered product segment held a dominant presence in the advanced therapy medicinal products market in 2024. The production of biologically active tissues incorporates scaffolding methods that stimulate both new cell development and restoration of harmed tissues. Bioprinting combined with 3D cell culture systems has caused unprecedented improvements in tissue structure development within this sector. The market forecasting predicts fast growth for tissue-engineered therapies, as numerous orthopedic dermatologic and cardiovascular conditions require tissue regeneration. Moreover, the tissue engineering sector expects to propel regenerative medicine forward, as emerging research on both stem cells and biomaterials provides hope to chronically injured patients.
The gene therapy segment is expected to grow at a significant pace in the market during the forecast period of 2025 to 2034, as it has the capability to treat genetic disorders in its original location. Through gene therapy, doctors focus on redesigning patient cellular genes to fight diseases or stop them from occurring, which provides enduring medical outcomes beyond standard therapeutic methods. The growth of gene therapy relies on essential advancements in CRISPR alongside other gene-editing technologies that allow exact genetic material modifications. The regulatory approvals led to investment growth between pharmaceutical companies and research institutions. Furthermore, pharmaceutical companies will develop treatments for uncommon hereditary illnesses to further facilitate the segment in the coming years.

By Therapy
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
December 2024
December 2024
December 2024
February 2025