September 2024
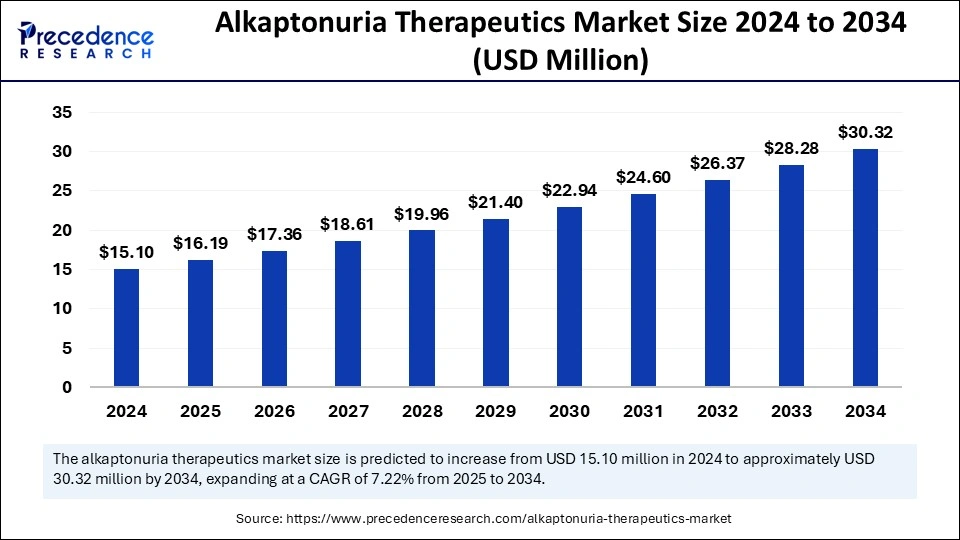
The global alkaptonuria therapeutics market size is calculated at USD 16.19 million in 2025 and is forecasted to reach around USD 30.32 million by 2034, accelerating at a CAGR of 7.22% from 2025 to 2034. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global alkaptonuria therapeutics market size accounted for USD 15.10 million in 2024 and is predicted to increase from USD 16.19 million in 2025 to approximately USD 30.32 million by 2034, expanding at a CAGR of 7.22% from 2025 to 2034. The market growth is attributed to increasing research efforts, rising adoption of targeted therapies, and expanding rare disease awareness programs, driving early diagnosis and improved treatment accessibility.
The therapeutic landscape for rare diseases experiences transformation through Artificial Intelligence systems as they speed up drug research and enhance clinical trials and diagnostic tests. Machine learning programs help scientists discover alkaptonuria drug candidates through automatic screening, which decreases development periods in the process. Such models examine large genetic study and biochemical pathway databases to discover new therapeutic targets that enhance treatment strategy precision.
Predictive analytics and AI-based imaging solutions help medical professionals detect concealed early signs of diseases that normal approaches miss. Research shows that the continuing development of AI expands its capability to drive targeted medicine innovations specifically for alkaptonuria treatment, which results in improved patient clinical results.
The approval from regulatory authorities such as the European Medical Agency (EMA) and European Commission has dramatically improved alkaptonuria management by providing a therapeutic solution that stops the disease's progression. Furthermore, the future expansion of the alkaptonuria therapeutics market is projected from sustained attention to rare disease investigation and treatment development initiatives.
Nitisinone is a promising active pharmaceutical ingredient for the treatment of alkaptonuria. It has led to substantial market expansion in the field of alkaptonuria therapeutics since its approval as a medical treatment option. Nitisinone blocks the activity of 4-hydroxyphenylpyruvate dioxygenase (HPPD) enzyme to lower homogentisic acid accumulation, thus minimizing the tissue damage in alkaptonuria.
| Report Coverage | Details |
| Market Size by 2034 | USD 30.32 Million |
| Market Size by 2025 | USD 16.19 Billion |
| Market Size by 2024 | USD 15.10 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 7.22% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Drug Type, Route of Administration, Patient Age Group, Distribution Channel, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Expanding awareness and advancements in early diagnosis
Growing awareness campaigns and diagnostic advancements are anticipated to improve early detection rates, enabling timely intervention. The expansion of awareness initiatives, together with enhanced diagnostic capabilities, results in earlier alkaptonuria diagnoses, thus enabling immediate medical intervention. The use of genetic screening programs, together with next-generation sequencing technologies, helps medical professionals detect alkaptonuria before serious complications occur.
Healthcare providers, along with patient advocacy groups and rare disease organizations, work together to teach people about alkaptonuria signs and its stage development. Government programs for newborn screening of metabolic diseases enhance diagnostic abilities, which enables health providers to intervene at an earlier stage. These advancements demonstrate worldwide dedication towards early detection and care of rare genetic conditions that benefit patients with alkaptonuria.
Populations Suffering from Rare Disorders, 2022-2024
| Year | Estimated Global Population |
| 2022 | 8.0 billion |
| 2023 | 8.1 billion |
| 2024 | 8.2 billion |
Scarcity of approved therapies
The scarcity of approved therapies is expected to challenge treatment accessibility, leaving patients with limited pharmacological options. Pharmaceutical organizations refrain from investing in treatments for ultra-rare diseases because these conditions yield minimal potential for the alkaptonuria therapeutics market, which slows down discoveries in therapeutic development. Approval processes for rare disease treatments managed by regulatory agencies demand extensive clinical validations that extend the duration required for commercialization.
Increasing research and development initiatives
Increasing investments in research and development activities are projected to drive advancements in targeted therapies for alkaptonuria, which further creates immense opportunities for the players competing in the market. Pharmaceutical organizations, together with academic research institutions, develop enzyme replacement treatments and gene-editing methods, and drug repurposing techniques to heal metabolic flaws in alkaptonuria patients.
Clinical evaluations of nitisinone therapy and new treatment compounds are continuing to expand for the purpose of reducing homogentisic acid accumulation and long-term complications prevention. New research on biochemical mechanisms enables healthcare providers to enhance therapy through precise medical methods that yield enhanced patient healing results. Alkaptonuria research receives increased emphasis on developing innovative treatments due to extensive research funding alongside promising clinical study results.
The nitisinone (p-hydroxyphenyl-pyruvate dioxygenase inhibitor) segment dominated the alkaptonuria therapeutics market during the forecasting period. The therapeutic agent Nitisinone shows effectiveness in homogentisic acid reduction, as this substance functions as the main factor in alkaptonuria-related medical complications. The European Medicines Agency (EMA) formally approved Nitisinone as the first alkaptonuria therapy for its effectiveness and broader use of the treatment. Monitored clinical trials showed that nitisinone therapy reduced homogentisic acid in patients' urine samples, thereby confirming its medical benefit.
The dietary supplements segment is expected to grow at the fastest rate during the forecast period of 2025 to 2034. The growing trend is for patients, together with healthcare professionals, to center their disease management approach on nutritional practices alongside supplementation. Patients who take dietary supplements with vitamin C and antioxidants face a potential benefit of slowing the disease progression rate through their antioxidant effects on alkaptonuria-derived oxidative stress. The Council for Scientific and Industrial Research (CSIR) India suggests pharmacological advancements boost the demand for supplementary medicine as well as preventable healthcare services. The wide accessibility of dietary supplements that are purchased without a prescription contributes to their expected market dominance, as they reach a high number of patient communities.
The oral segment held the largest revenue share of the alkaptonuria therapeutics market during the forecasting period. The oral pharmaceutical substance reduces the accumulation of homogentisic acid, which creates complications in alkaptonuria patients. The production expenses of oral dosage forms stay lower than those of parenteral or topical products as they become available to broader groups of patients. The segment has gained further market strength through the regulatory approval of nitisinone as an oral medication.
The parenteral segment is anticipated to grow with the highest CAGR in the studied years, owing to the ongoing enzyme replacement therapy research and development of gene therapies, which probably result in therapeutic delivery methods that require intravenous or subcutaneous administration. Medical delivery methods provide a direct pathway for therapeutic substances into the bloodstream, which enables swift drug effects with accurate dosing capability. Furthermore, the expanding use of sophisticated treatment approaches as they advance through the clinical testing process and gain regulatory clearances further fuels the segment in the coming years.
The adult segment dominated the alkaptonuria therapeutics market due to the symptoms generally appearing after age thirty, resulting in increased diagnosis and treatment of these patients. Adults with homogentisic acid buildup in their system develop dark-pigmented connective tissues referred to as ochronosis, along with arthritic conditions that affect weight-bearing joints most severely. Medical intervention becomes essential as these progressively worsening symptoms increase the therapy requirements in the affected population.
The pediatric segment is projected to expand rapidly in the coming years. The identification of alkaptonuria through genetic screening and early diagnostic techniques shifts the market towards children, as these techniques allow doctors to identify alkaptonuria before serious symptoms occur. Early treatment methods, which combine nutritional adjustments and medical medications, work to halt disease progression or stop its advancement from the beginning. Healthcare professionals, along with parents, increase their early detection knowledge as the market demand for paediatric therapeutic solutions is growing.
The hospital pharmacies segment held the largest share of the alkaptonuria therapeutics market during the forecasting period. The specialized treatments for alkaptonuria require healthcare professional supervision, thus leading to hospital pharmacies' market dominance. Hospital pharmacies keep a complete stock of unlikely disease medications to ensure quick access for their patient population. Hospital integrated care systems allow doctors to share information directly with pharmacy staff, which leads to improved patient outcomes and medication usage performance. Furthermore, the hospital pharmacies deliver specific patient education together with support services to assist individuals with managing their challenging condition of alkaptonuria.
The online pharmacies segment is projected to grow at the fastest rate in the future years, owing to the increasing growth rates of online pharmacies' availability. The merging of digital health platforms with home delivery convenience services causes this transition in the future. People living in remote locations gain better access to medications through online pharmacy services since these platforms distribute special pharmaceuticals unavailable at standard retail outlets. Online research allows patients to gain essential knowledge about their care options and assessment of prices, which helps them make smarter healthcare decisions. Furthermore, online pharmacies receive greater trust from consumers, as the regulatory organizations now establish safety guidelines for these virtual pharmaceutical facilities.
North America led the alkaptonuria therapeutics market, capturing the largest share in 2024, as the region holds advanced healthcare facilities together with robust regulatory structures and substantial funding for rare disease therapeutic research. The National Institutes of Health (NIH) maintains its ongoing support for metabolic disorder research, specifically for alkaptonuria, which advances specific treatment methods. It allocates approximately USD 3.5 billion annually to rare diseases research, facilitating the development of novel therapies for conditions.
Genetic screening programs and metabolic clinics located in the United States and Canada enable patients to get diagnosed early with the opportunity for timely treatments. The awareness campaigns initiated by rare disease organizations, including the National Organization for Rare Disorders (NORD), have improved both disease education alongside treatment accessibility for patients. North America maintained its position as leader in the alkaptonuria therapeutics market of 2024 due to these multiple market-promoting factors.
Asia Pacific is projected to host the fastest-growing alkaptonuria therapeutics market in the coming years. Genetic screening expansion throughout these countries improves alkaptonuria early diagnosis, which leads to faster interventions. The growth of patient access to treatment, together with therapeutic advancements, stems from government funding programs that back rare disease research. Furthermore, the partnership between international pharmaceutical businesses and local healthcare organizations creates stronger access to specialized medicines, which further facilitates the market in this region.
According to the NIH 2024 report, countries such as China and India have experienced annual healthcare expenditure growth rates of 8% and 7%, respectively, between 2019 and 2023, enhancing healthcare infrastructure and access to treatments.

By Drug Type
By Route of Administration
By Patient Age Group
By Distribution Channel
By Region
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
September 2024
August 2024
October 2024
March 2025