March 2024
The global atherectomy devices market size accounted for USD 1.12 billion in 2025 and is forecasted to hit around USD 2.27 billion by 2034, representing a CAGR of 8.12% from 2025 to 2034. The North America market size was estimated at USD 440 million in 2024 and is expanding at a CAGR of 8.11% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global atherectomy devices market size was calculated at USD 1.04 billion in 2024 and is predicted to increase from USD 1.12 billion in 2025 to approximately USD 2.27 billion by 2034, expanding at a CAGR of 8.12% from 2025 to 2034. The market is driven by atherectomy device innovations that include technological advancements, such as miniaturization and improved imaging modalities, enhancing precision and efficacy in plaque removal.
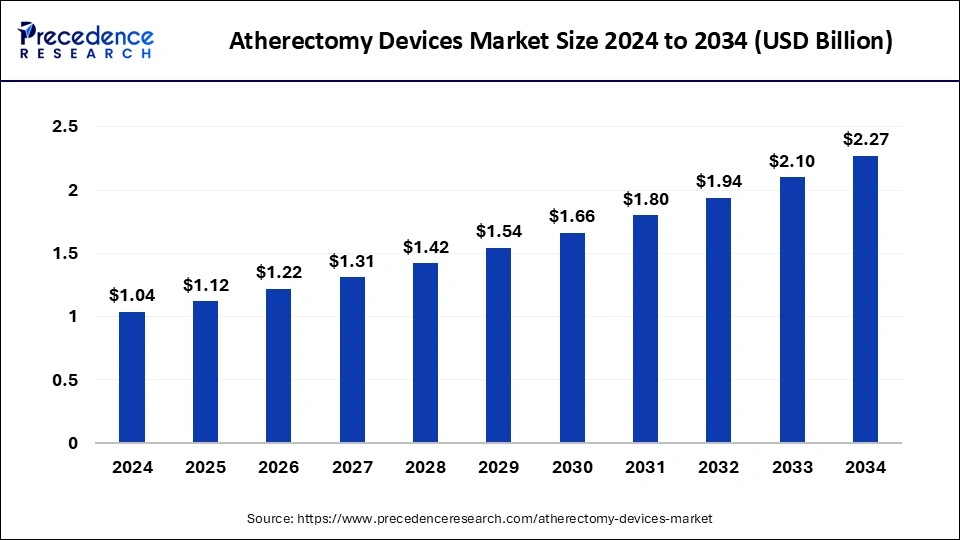
The U.S. atherectomy devices market size was exhibited at USD 330 million in 2024 and is projected to be worth around USD 730 million by 2034, growing at a CAGR of 8.26%.
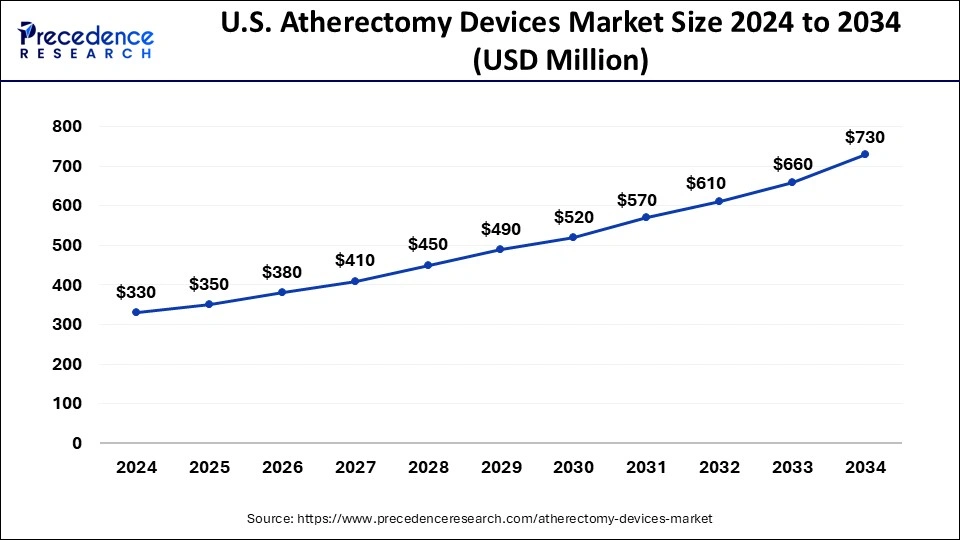
North America led the global market with the highest market share of 42% in 2024. Atherectomy devices have seen significant utilization and dominance in North America, particularly in the United States of America (USA) and Canada. The USA boasts advanced healthcare infrastructure and extensive research and development capabilities, driving the adoption of atherectomy devices for the treatment of atherosclerosis. Similarly, Canada benefits from a well-established healthcare system and a high prevalence of cardiovascular diseases, contributing to the widespread use of these devices.
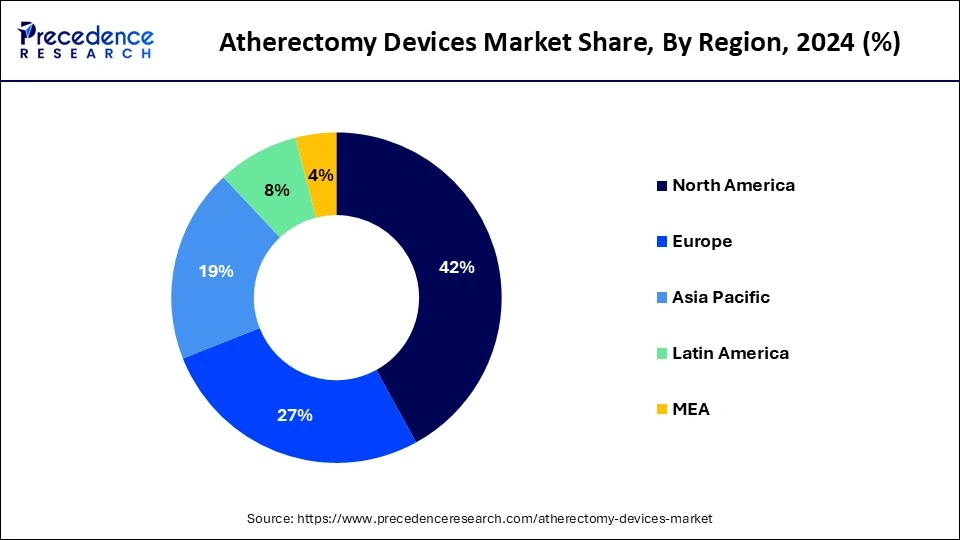
Asia-Pacific is expected to witness growth at a significant rate in the atherectomy devices market during the forecast period of 2025-2034. Asia Pacific region is experiencing rapid growth and emergence as a key market for atherectomy devices. Countries like China, India, Japan, and South Korea are witnessing increasing adoption of these devices due to rising healthcare awareness, improving access to advanced medical technologies, and a growing burden of cardiovascular diseases. As healthcare infrastructure continues to develop and awareness of minimally invasive treatment options expands, the Asia Pacific region is expected to play a significant role in the global market for atherectomy devices.
Atherectomy devices are specialized medical instruments used to remove plaque buildup from arteries, particularly in the treatment of atherosclerosis. Atherosclerosis is a condition where plaque, made up of cholesterol, fat, calcium, and other substances, accumulates on the inner walls of arteries, leading to narrowing and hardening of the arteries. Atherectomy procedures are minimally invasive alternatives to traditional surgical methods, such as bypass surgery, and are often performed to restore blood flow in arteries affected by atherosclerosis.
There are several types of devices, each designed for specific applications and preferences of clinicians. Rotational devices use a rotating burr or blade to shave away plaque from the artery walls, while directional devices employ a cutting mechanism to remove plaque in a controlled manner. Laser atherectomy devices use laser energy to vaporize plaque, and orbital atherectomy devices utilize a unique orbital mechanism to sand down plaque. Each type of device has its advantages and may be chosen based on factors such as the location and severity of plaque buildup and the patient’s overall health condition.
A therectomy devices have benefited from continuous technological advancements, leading to the development of more efficient and precise tools for plaque removal in arteries. Innovations such as improved rotational mechanisms, enhanced visualization capabilities, and miniaturization of devices have contributed to greater efficacy and safety in atherectomy procedures. These advancements enable physicians to navigate through complex arterial structures with increased precision, facilitating thorough plaque removal while minimizing damage to surrounding tissues.
The growth of the atherectomy devices market is bolstered by ongoing clinical research and evidence supporting their effectiveness and safety in treating arterial disease. Clinical trials and studies provide valuable insights into the outcomes and benefits of atherectomy procedures, helping to establish their role in the management of various arterial conditions. As more evidence emerges demonstrating favorable patient outcomes and reduced procedural complications, confidence in the use of atherectomy devices among healthcare providers grows, driving further adoption and utilization.
Comprehensive physician training and education programs play a crucial role in the adoption and utilization of atherectomy devices. Manufacturers invest in training initiatives to educate healthcare professionals on the proper use of atherectomy systems, procedural techniques, and patient selection criteria. By equipping physicians with the necessary knowledge and skills, these training programs empower them to confidently incorporate atherectomy into their clinical practice, expanding access to this minimally invasive treatment option for patients with arterial disease.
The atherectomy devices market continues to evolve with expanded indications and applications, allowing for their utilization in a broader range of clinical scenarios. Manufacturers continually seek regulatory approvals for new indications and expand the capabilities of existing devices to address evolving clinical needs. This expansion of indications, coupled with advancements in device design and technology, enhances the versatility and effectiveness of atherectomy procedures, positioning them as a valuable treatment option for an increasing number of patients with arterial disease.
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 8.12% |
| Market Size in 2025 | USD 1.12 Billion |
| Market Size by 2034 | USD 2.27 Million |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Devices Type, Application, and End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Drivers
Technological innovation
Advancements in medical technology have revolutionized the atherectomy devices market. Miniaturization, improved imaging modalities, and the development of novel materials have enabled the creation of increasingly sophisticated atherectomy devices. For example, the integration of advanced imaging techniques such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT) allows for real-time visualization of arterial structures, guiding precise interventions.
The miniaturization of devices has facilitated the use of minimally invasive procedures, reducing patient discomfort and recovery time. Continued innovation in device design, including the incorporation of robotics and artificial intelligence, holds promise for further enhancing the efficacy and safety of the products and services of the atherectomy devices market.
Deeper understanding of arterial biomechanics
Atherosclerosis is not merely a passive buildup of plaque within arteries but a dynamic process influenced by arterial biomechanics. Research into the mechanical forces acting on arterial walls, such as shear stress and cyclic strain, has revealed their profound effects on endothelial function, plaque development, and vulnerability. This deeper understanding has informed the design of atherosclerosis devices that not only remove plaque but also modulate biomechanical factors to promote arterial healing and stability.
Devices capable of delivering localized drug therapies or modulating blood flow patterns can help prevent restenosis and thrombosis. By integrating biomechanical principles into device design and treatment strategies, clinicians can tailor interventions to address the specific biomechanical challenges posed by individual patients’ arterial lesions, leading to improved outcomes in atherosclerosis management.
Restraint
Regulatory hurdles in navigating complex approval processes and safety standards for atherosclerosis devices
Navigating the complex landscape of regulatory approvals poses a significant restraint in the development and deployment of atherosclerosis devices. Manufacturers must adhere to stringent safety standards and demonstrate the efficacy of their devices through rigorous clinical trials, adding time and cost to the development process. Regulatory agencies, such as the FDA in the United States and the European Medicines Agency (EMA) in Europe, require comprehensive data on device performance, including safety profiles and long-term outcomes, before granting market approval.
Additionally, evolving regulatory requirements and guidelines further compound the challenge, necessitating continuous adaptation and compliance. Delays in regulatory approvals or failure to meet stringent criteria can significantly impede the availability of new atherosclerosis devices, limiting options for patients and clinicians and hindering advancements in treatment modalities for arterial disease.
Emerging therapeutic modalities on expanding treatment options for atherosclerosis management
One significant opportunity in the atherectomy devices market lies in the exploration and development of emerging therapeutic modalities. Beyond traditional atherectomy procedures, researchers are investigating innovative approaches such as gene therapy, targeted drug delivery, and immunomodulation to address the underlying mechanisms of arterial disease.
Gene therapy holds promise for delivering therapeutic genes directly to affected arteries, promoting plaque stabilization and regression. Similarly, targeted drug delivery systems enable precise delivery of therapeutic agents to diseased arterial segments, minimizing systemic side effects and enhancing efficacy. Furthermore, immunomodulatory strategies aim to modulate the immune response within arterial walls, reducing inflammation and promoting plaque stability. By leveraging these emerging therapeutic modalities, atherosclerosis devices can offer more tailored and effective treatments, potentially improving outcomes for patients with arterial disease.
Integration of digital health technologies on enhancing monitoring and personalized management of atherosclerosis
Another critical aspect of the atherectomy devices market is patient engagement and self-management, which can be enhanced through interactive educational resources, real-time feedback, and support networks, empowering individuals to take an active role in managing their arterial health. Overall, the integration of digital health technologies into atherosclerosis devices holds promise for improving patient outcomes, enhancing clinical decision-making, and reducing healthcare costs.
Another critical aspect of the atherectomy devices market is patient engagement, and self-management that can be enhanced through interactive educational resources, real-time feedback, and support networks, empowering individuals to take an active role in managing their arterial health. Overall, the integration of digital health technologies into atherosclerosis devices holds promise for improving patient outcomes, enhancing clinical decision-making, and reducing healthcare costs.
The drug-coated balloons (DCBs) segment dominated the atherectomy devices market with the largest share in 2024. DCBs Catheters. DCBs have gained popularity due to their ability to deliver anti-proliferative drugs directly to the arterial wall during balloon angioplasty, reducing restenosis rates and the need for additional stents.
The intravascular ultrasound (IVUS) catheter segment is expected to show the fastest growth in the atherectomy devices market during the forecast period. IVUS catheters provide real-time, high-resolution imaging of the arterial lumen and vessel wall, aiding in lesion assessment, stent sizing, and optimization of stent deployment. Both technologies have contributed to improved procedural outcomes, reduced complication rates, and enhanced patient care in the management of atherosclerotic disease.
The peripheral artery disease (PAD) treatment devices segment dominated the atherectomy devices market in 2024. Recent advancements in PAD treatment devices have focused on enhancing efficacy and durability while minimizing invasiveness. Technologies such as drug-coated balloons and drug-eluting stents have shown promising results in reducing restenosis rates and improving the long-term patency of treated arteries. Additionally, the development of atherectomy devices with improved navigation and tissue removal capabilities has expanded treatment options for complex PAD lesions, particularly in cases where traditional angioplasty or stenting may not be feasible.
The coronary artery disease (CAD) intervention device segment shows notable growth in the atherectomy devices market during the forecast period. In the realm of CAD intervention devices, there has been a noteworthy emphasis on enhancing precision and safety during percutaneous coronary interventions (PCIs). Innovations such as bioresorbable vascular scaffolds (BVS) and drug-eluting stents with biocompatible coatings aim to reduce the risk of late stent thrombosis and improve vascular healing. Furthermore, the integration of intravascular imaging technologies, such as optical coherence tomography (OCT) and intravascular ultrasound (IVUS), has enabled more accurate lesion assessment and optimized stent placement, leading to improved procedural outcomes and long-term clinical success rates.
The catheterization labs and interventional radiology departments segment dominated the atherectomy devices market in 2024. cardiac catheterization labs and interventional radiology departments have seen increased usage of atherosclerosis devices. Cardiac catheterization labs specialize in diagnosing and treating cardiovascular conditions, including atherosclerosis, through minimally invasive procedures.
The interventional radiology department segment is expected to witness a significant rate of expansion during the forecast period. Interventional radiology departments offer expertise in image-guided interventions, making them well-suited for atherosclerosis device placement and monitoring. Both settings provide advanced equipment and skilled personnel, facilitating the precise placement and monitoring of atherosclerosis devices to manage arterial disease effectively. This trend reflects the growing importance of minimally invasive techniques and interdisciplinary collaboration in the treatment of atherosclerosis.
By Devices Type
By Application
By End-user
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
March 2024
January 2025
January 2025
February 2025