February 2025
The global autologous cell therapy market size is calculated at USD 11.43 billion in 2025 and is forecasted to reach around USD 53.73 billion by 2034, accelerating at a CAGR of 18.86% from 2025 to 2034. The North America autologous cell therapy market size surpassed USD 4.20 billion in 2024 and is expanding at a CAGR of 18.90% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global autologous cell therapy market size was garnered at USD 9.55 billion in 2024 and is expected to hit around USD 53.73 billion by 2034, growing at a CAGR of 18.86% during the forecast period from 2025 to 2034.
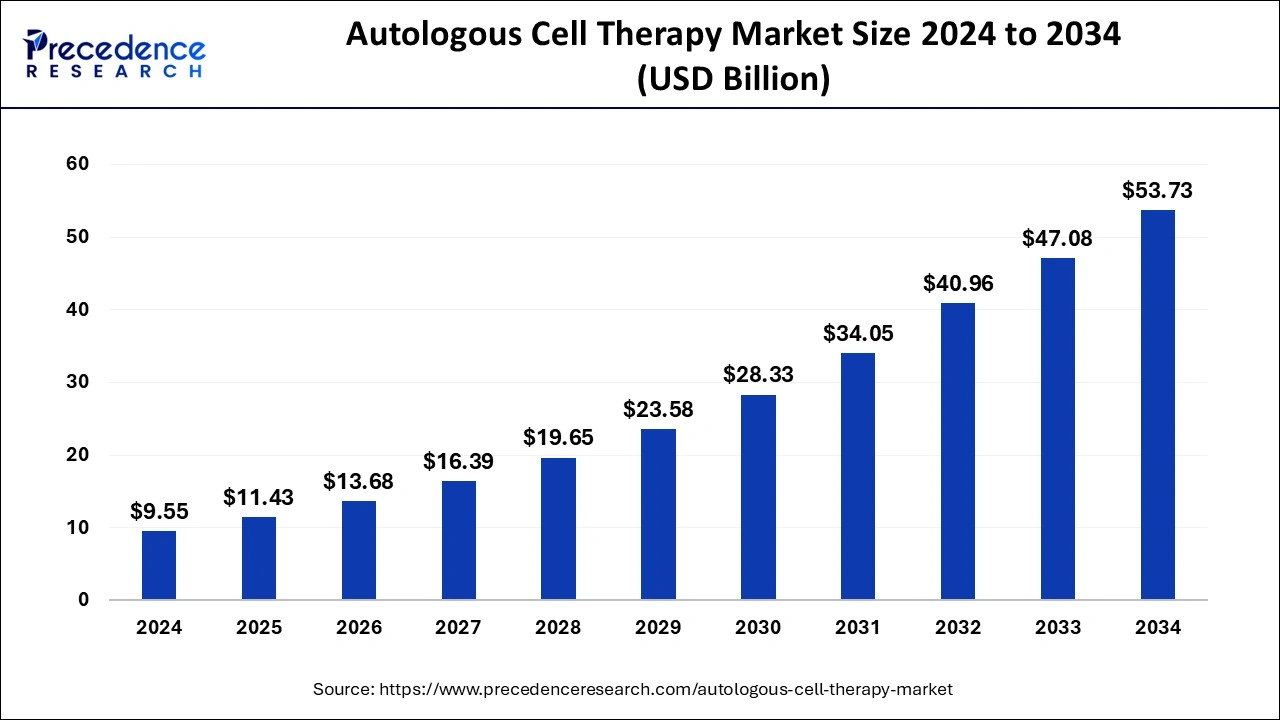
The U.S. autologous cell therapy market size reached USD 2.90 billion in 2024 and is anticipated to be worth around USD 17.14 billion by 2034, poised to grow at a CAGR of 19.44% from 2025 to 2034.
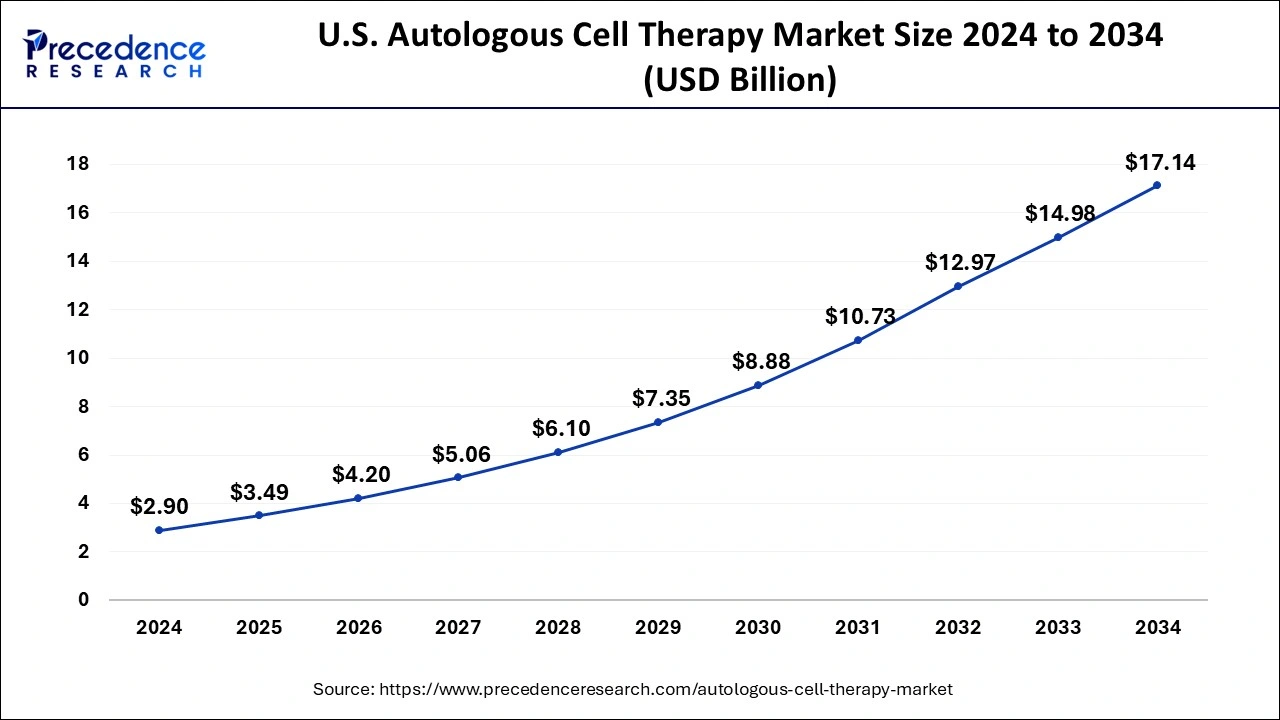
North America is expected to dominate the market during the forecast period. The growth in the region is attributed to the growing prevalence of chronic diseases such as cardiovascular, cancer, autoimmune disease, and others. For instance, in the United States, more than 1.9 million new instances of cancer are anticipated to be identified in 2022, according to a report by the American Cancer Society. In addition, 57% of cancer patients in the United States are 65 or older, and 80% of cancer patients there are.
As a result, the need for diagnostics and treatment is strong in the nation due to the high prevalence of cancer and the growing number of geriatric patients, which is likely to fuel the expansion in the region. Furthermore, the increasing prevalence of neurodegenerative disease in the countries like the United States is another prominent factor that propels the market expansion over the forecast period.
According to the Parkinson’s Foundation, in the United States, almost a million people have Parkinson’s disease. By 2030, it is anticipated to reach 1.2 million. Parkinson’s disease is the most common neurodegenerative disease after Alzheimer’s in the US. Thus, this is expected to propel market growth in the region.
The Asia Pacific is growing at the highest CAGR during the forecast period. The growing prevalence of cancer in countries like India and China is expected to drive market expansion in the region. For instance, as per the US Department of Health and Human Services (HHS), 14,61,427 incident cases of cancer were determined to be the projected total in India for 2022. In India 1 out of 9 people has a lifelong risk of developing cancer. The most common cancer in men and women were lung and breast cancer. According to the estimates, there will be 12.8% more cancer instances in 2025 than there were in 2020. Moreover, the growing heart failure in the countries like India and China is further driving the demand for ACT therapy in the region.
Autologous cell therapy (ACT) is a cutting-edge therapeutic technique that utilizes a donor’s cell after they have been enlarged and cultured outside of its body. The reduction of hazards from systematic immunological reactions, bio-incompatibility, and disease transmission associated with grafts or cells not grown from the individual are benefits of a technique like this. Bioengineering skin substitutes, promoting wound healing, reducing chronic inflammation, treating burns, and pressure ulcers, and enhancing surgical healing have all been accomplished so far with the use of these types of therapy. This therapy is also used for cosmetic enhancement or corrective surgery.
The use of ACT for cosmetic surgery or corrective enhancement is also gaining acceptance as an appropriate kind of treatment because it has been demonstrated to lower the likelihood of rejection and to provide benefits that persist longer than those of conventional treatments. To improve the process by which ageing or damaged tissues are repaired, this sort of treatment is currently the subject of extensive research with the aim that one day it may be able to take place of traditional plastic surgery.
The market is driven by various factors including the prevalence of chronic diseases such as cancer, cardiovascular, Alzheimer and Parkinson’s. Moreover, these therapies are also used for cosmetic enhancement or corrective surgery which provides an enormous opportunity for market expansion during the forecast period. Furthermore, the growing number of approvals by the regulatory bodies also propel the market growth over the forecast period.
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 18.86% |
| Market Size in 2025 | USD 11.43 Billion |
| Market Size by 2034 | USD 53.73 Billion |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | By Source, By Application, and By End-Use |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Growing investment in cell therapy
The growing investment in cell therapy is expected to drive the market growth over the forecast period. For instance, as part of a deal with two biotechnology companies and seven public hospitals, LFB is investing in a significant cell therapy medication production initiative. This project, known as C4C, is sponsored by OSEO (a French organization dedicated to supporting creative ventures), and it aims to commercialize cell therapy solutions. This project, which is being managed by LFB subsidiary CELLforCURE, realizes the goal of creating a French cell treatment business in which it will play a significant role.
The C4C project intends to establish the first technical support unit for industrial cell therapy in France, dedicated to the mass production of allogeneic and autologous cell therapy products in conformity with laws controlling advanced therapeutic pharmaceuticals. Thus, this is expected to dominate the market over the forecast period.
Lack of cost-effective and quality product
Any biotechnological technique must meet commercial criteria, which demand cost-effectiveness, suitability for purpose, and either ease of production or accessibility to the process and results. To achieve desired cell development while minimizing expenses, cell cultivation frequently uses the use of undefined components, such as animal serum, in culture media.
The inability to precisely specify the nature and expression of the biomolecules and other variables present, as well as the possibility that cells would become contaminated with or altered by animal-derived products, are the potential hazards. The use of numerous growth factors and extrinsic conditions, such as temperature, CO2, and humidity, which may affect the metabolic activity of the cell and, as a result, the function, specificity, and efficacy, are other aspects of tissue culture that are being questioned. Thus, this is expected to hamper the market expansion during the forecast period.
Increasing FDA approvals
The increasing FDA approvals in autologous cell therapy are expected to provide an attractive opportunity for the growth of the market during the forecast period. For instance, in June 2022, the US Food and Drug Administration (FDA) approved Breyanzi (lisocabtagene maraleucel), a CD19-directed chimeric antigen receptor (CAR) T-cell therapy, for the treatment of adult patients with large B-cell lymphoma (LBCL), including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B. In addition, the FDA has approved Yescarta (axicabtagene ciloleucel) CAR T-cell therapy for adult patients with large B-cell lymphoma who are refractory to first-line chemoimmunotherapy, according to an announcement from Kite, a Gilead Company. Thus, the growing FDA approvals are expected to provide a lucrative opportunity for market growth.
Based on the source, the global autologous cell therapy market is segmented into Epidermis, Bone Marrow, Mesenchymal stem cells, Haematopoietic Stem Cells, Chondrocytes and Others. The bone marrow segment is expected to capture the largest revenue share over the forecast period. In ACT, various disorders have been effectively treated using bone marrow. One of the main sources for cell therapy is this method, in which the patient’s healthy stem cells are extracted from the bone marrow or blood and stored before the duration of treatment. As an alternative, ACT using bone marrow has some advantages over using donor stem cells.
The Mesenchymal stem cells segment is expected to grow at the fastest rate over the forecast period. The growth in the segment is attributed to the application in preclinical and clinical tissue regeneration to treatment for immune disease.
Based on the application, the market is bifurcated into Cancer, Cardiovascular Disorders, Neurodegenerative Disorders, Autoimmune Disorders, Orthopedics, Wound Healing, and Others.
The cancer segment is expected to dominate the market over the forecast period. The segment growth is attributable to the rising prevalence of cancer across the globe. The American Cancer Society estimates that there will be 1.9 million new cancer diagnoses and 608,570 cancer-related deaths in the United States in 2021. With the rising incidence of cases and demand for therapy, autologous cell therapy is projected to find its greatest demand in cancer applications. Moreover, in August 2022, Anixa Biosciences announced that it has given the first dose of its FSHR-targeted autologous cell treatment to an ovarian cancer patient as part of a Phase I trial.
The cardiovascular segment is expected to grow at a significant rate over the forecast period. The growth in the segment is owing to the increasing incidence of heart failure in developed as well as developing economies. According to the American Heart Association, globally, CVD was estimated to be responsible for 19.1 million deaths in 2020. The age-adjusted mortality rate was 239.8 per 100,000 people. The adjusted prevalence rate for age was 7354.1/100,000. Thereby, driving the segment growth over the forecast period.
Based on the end-use, the market is segmented into Hospitals & Clinics, Ambulatory Centers, Academics & Research, and Others. The Hospitals & Clinics segment is expected to capture the largest revenue share during the forecast period. The growth in this segment is owing to the rising prevalence of various diseases across the globe including cancer, cardiovascular, autoimmune and others, this disease required treatment in hospitals and clinics. Thereby, driving the market growth over the forecast period.
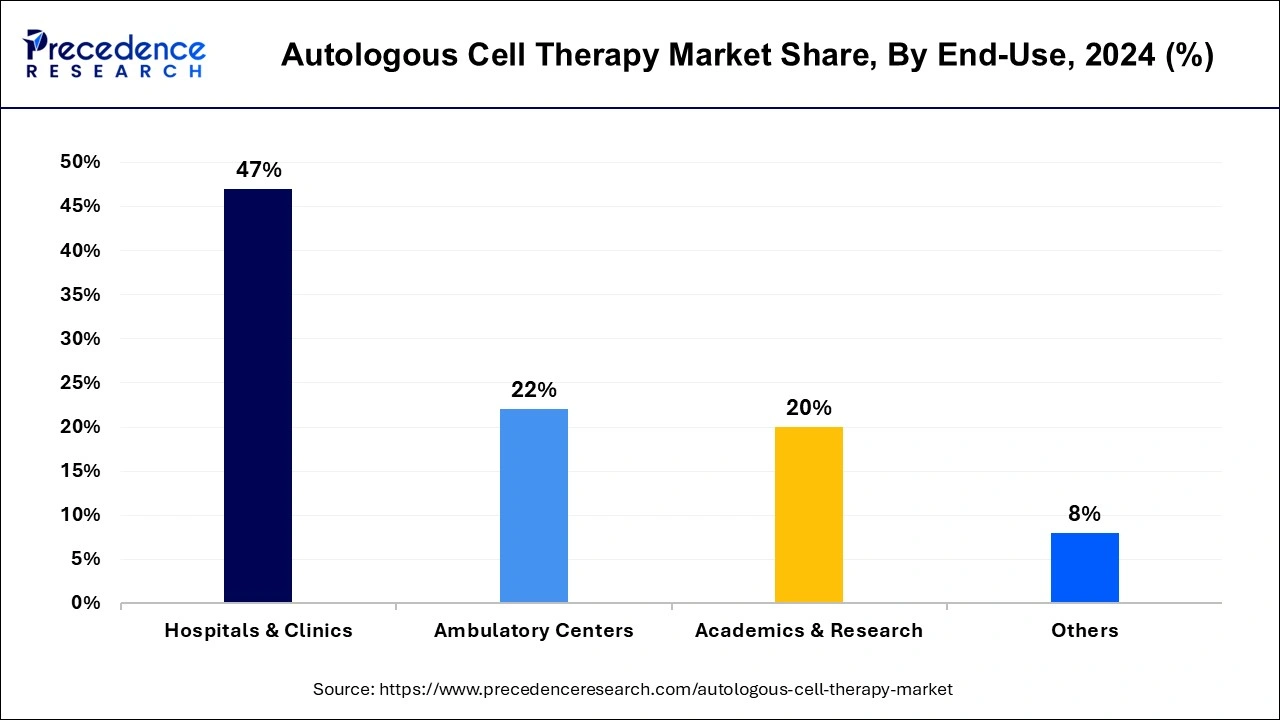
Academics & Research is growing substantially during the forecast period. A lot of research is being done on ACT, which is attracting growing interest globally. Utilizing live cells to cure and prevent disease is a new scientific area. The research is primarily done on mesenchymal stem cells, chondrocytes, bone marrow, and others. Thus, this is expected to drive market growth over the forecast period.
By Source
By Application
By End-Use
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
February 2025
January 2025
January 2025
January 2025