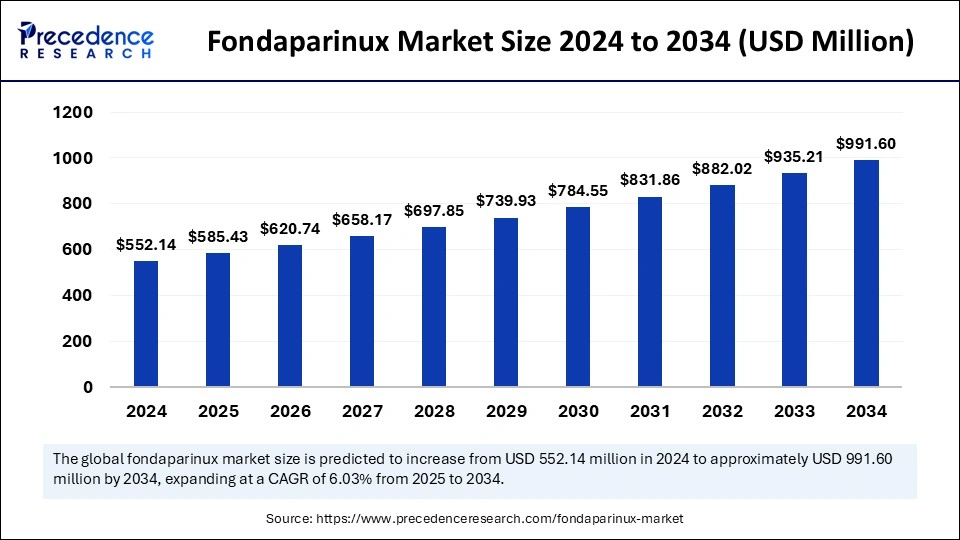
The global fondaparinux market size is calculated at USD 585.43 million in 2025 and is forecasted to reach around USD 991.60 million by 2034, accelerating at a CAGR of 6.03% from 2025 to 2034. The North America market size surpassed USD 209.81 million in 2024 and is expanding at a CAGR of 6.16% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global fondaparinux market size accounted for USD 552.14 million in 2024 and is predicted to increase from USD 585.43 million in 2025 to approximately USD 991.60 million by 2034, expanding at a CAGR of 6.03% from 2025 to 2034. The market is driven by an aging population, rising cardiovascular diseases, R&D advancements, strengthening healthcare infrastructure, and regulatory approval benefits.
Integrating artificial intelligence in healthcare tools helps diagnose deep vein thrombosis and pulmonary embolism during the early stages. Healthcare personnel use improved risk assessments based on AI predictive models to control anticoagulation therapy while reducing bleeding complications. The manufacturing process of pharmaceuticals becomes more efficient because AI achieves consistent quality inspections while maximizing production metrics.
AI infrastructure within healthcare facilities through electronic health records systems enables medical professionals to follow patient histories and understand treatment reactions, which enhances clinical decision quality. AI's integration in the fondaparinux market will boost market expansion while improving patient treatment and developing innovative anti-coagulant medication through research.
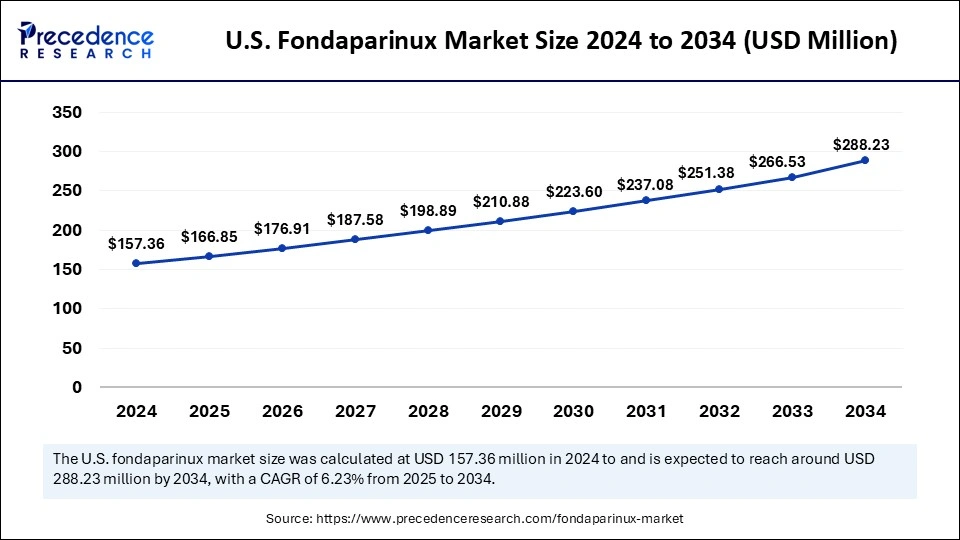
The U.S. fondaparinux market size was exhibited at USD 157.36 million in 2024 and is projected to be worth around USD 288.23 million by 2034, growing at a CAGR of 6.23% from 2025 to 2034.
North America Fondaparinux Market Trends
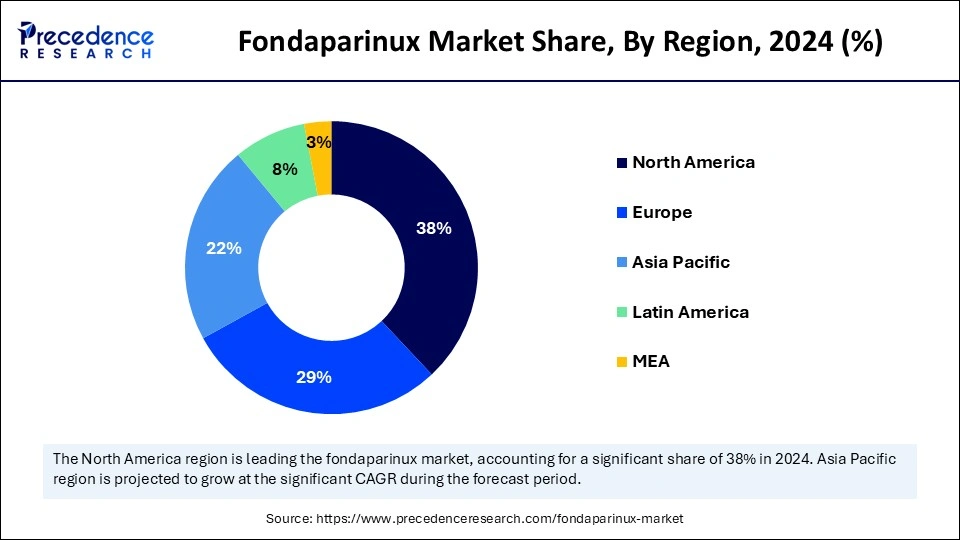
North America held the dominating share of the fondaparinux market in 2024. The strong market position results from its advanced healthcare systems, high medical technology usage, and powerful pharmaceutical capabilities. The healthcare facilities in this region effectively handle complex conditions, including venous thromboembolism (VTE) and pulmonary embolism (PE), which creates more demand for anticoagulant treatment with fondaparinux. Pharmaceutical organizations accelerate their new product launches in North America due to its fast approval procedure for medications.
The United States dominates the fondaparinux market sector because of its pharmaceutical industry and research and development function at a high level. The government initiatives and programs, patients have expanded access to vital treatments because these measures facilitate the more widespread adoption of fondaparinux.
Asia Pacific Fondaparinux Market Trends
Asia Pacific is expected to witness the fastest rate of growth during the predicted timeframe. The market expansion stems from cardiovascular disease growth rates of deep vein thrombosis (DVT) and pulmonary embolism (PE), which increase due to changes in lifestyle and population aging. Fondaparinux therapy continues to spread across India, China, and Japan because the governments support healthcare initiatives that enhance access to modern medical treatments.
China is experiencing rapid pharmaceutical research and development growth that leads to new treatment options. The growth of healthcare facilities across these countries will result in expanding fondaparinux availability, leading to swift market expansion in Asia Pacific.
Europe Fondaparinux Market Trends
The European market will experience continuous development due to advancements in healthcare systems together with economic developments. The comprehensive healthcare system of this region makes fundamental medications like fondaparinux available to all patients within its vast areas. Europe maintains fondaparinux as a central therapy for venous thromboembolic disease treatment because it delivers effective medical care to cardiovascular disease patients throughout the region. The European market is set for steady growth because of healthcare improvements and increasing rates of thromboembolic diseases.
Synthetic anticoagulant Arixtra (fondaparinux) derives from the heparin pentasaccharide sequence which contains its minimal antithrombin binding elements. Fondaparinux blocks blood clotting factor Xa activation to function as an indirect factor Xa inhibitor. Fondaparinux provides extended action against heparin with lower dosing frequencies which results in improved comfort for patients. The mechanism of fondaparinux prevents it from interacting with platelets which produces safer outcomes compared to heparin therapy. The medical use of fondaparinux focuses on treating deep vein thrombosis, pulmonary embolism, and superficial vein thrombosis.
Market growth for fondaparinux medicines continues to rise because of factors such as population aging worldwide, expanding cardiovascular disease cases, and ongoing development in medical science. An effective anticoagulation therapy remains essential for the elderly because aging makes them susceptible to blood clotting events such as deep vein thrombosis and pulmonary embolism. The improvement of healthcare frameworks in different areas has established better pathways for patients to obtain sophisticated therapeutic options.
| Report Coverage | Details |
| Market Size by 2034 | USD 991.60 Million |
| Market Size in 2025 | USD 585.43 Million |
| Market Size in 2024 | USD 552.14 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 6.03% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Type, Product, Distribution Channel, and Regions. |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Rising demand for anticoagulants in orthopedic surgery
The rising requirement for orthopedic surgical procedures acts as a primary market growth factor for fondaparinux. Fondaparinux anticoagulants serve as preventative blood clot medication during orthopedic surgical procedures that treat fractures, dislocations, and arthritis. Demand for these surgical procedures is on the rise because population aging, increased obesity statistics, and elevated sports incidence affect patient needs.
Orthopedic issues have become more common among elderly patients, and the increasing need for surgical interventions serves as a market driver for fondaparinux. The increase in orthopedic procedure demands stems directly from obesity because the condition causes joint pain and multiple joint-related medical problems. The rising number of sports-related injuries because of increased sports participation creates more demand for orthopedic surgical procedures.
Safety concerns and side effects
The main drawback of fondaparinux treatment is severe bleeding that leads to hemorrhagic stroke and causes organ damage, which makes the therapy unsuitable for widespread use, particularly in high-risk patients. Healthcare providers shun fondaparinux prescriptions due to safety concerns, which prevent them from giving this therapeutic option for anticoagulation monitoring treatment. Medical use of fondaparinux faces implementation challenges for patients experiencing renal impairment since the safety risks increase during treatment. The bleeding side effects of fondaparinux create a major obstacle to its market development.
Digital health platforms
Remote patient monitoring systems help healthcare providers implement digital health solutions, which provide accessible, efficient medical care through telemedicine services. Fondaparinux therapy patients receive outstanding value through mobile applications that provide real-time medication compliance tracking as well as quick dosage changes, leading to better patient results. On these platforms, medicine complications are reported to healthcare providers, and vital sign monitoring occurs, as well as medication alerts that drive anticoagulation treatment control improvements. The healthcare digital transformation, patients achieve specific treatments and better medical service availability.
The deep vein thrombosis (DVT) segment contributed the largest share of the fondaparinux market in 2024. Medical professionals observed rising demand for the effective treatment of fondaparinux because DVT cases continue to increase, specifically among older people and enduring health problems such as cancer. Older adult patients are more likely to develop deep vein thrombosis because their decreased mobility, reduced blood flow, and aging changes in their vascular system put them at risk.
Furthermore, chronic conditions such as cancer face higher risks of thrombosis due to the production of pro-thrombotic substances in the body. Fondaparinux serves as an established anticoagulant therapy in standard DVT patient care because it stops blood clot development.
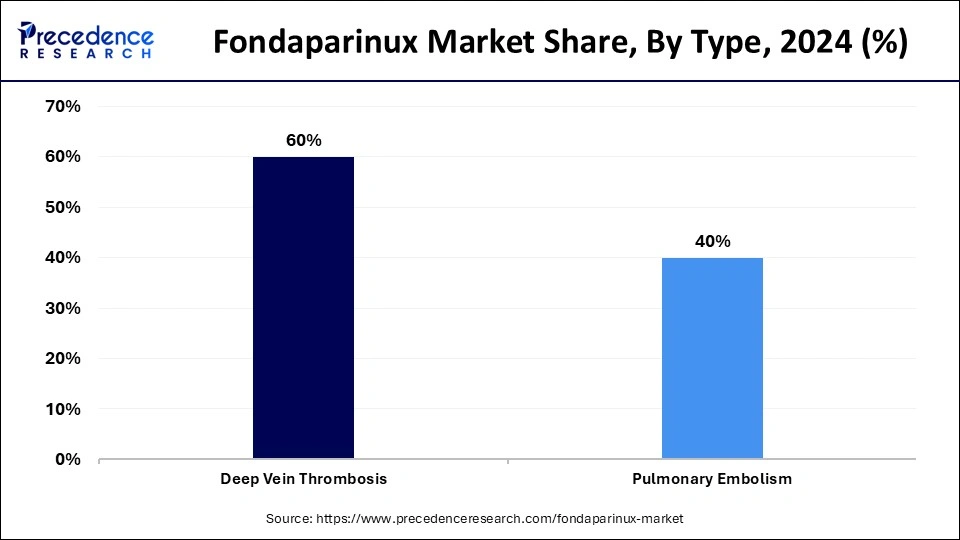
The pulmonary embolism segment is expected to grow considerably in the fondaparinux market. Blood clots develop more frequently among populations because age brings key risk factors, which include vascular changes in older individuals and reduced mobility during aging. PE development becomes more likely when patients suffer from obesity and smoke.
The increase in PE diagnoses stems from developed diagnostic methods, especially CT scans, so healthcare centers need effective treatments, which fondaparinux serves. The anticoagulant effect of this medication acts to control future blood clots and existing clots located within pulmonary arteries. Patients will increasingly use fondaparinux as prevention and individualized treatment for PE, rising in popularity, thereby solidifying its position in PE management.
The generic segment accounted for the largest share of the fondaparinux market. The healthcare community and patient population have shown major interest in generic fondaparinux because it offers effective treatment for thromboembolic diseases at reduced costs. The inpatient and outpatient departments have experienced rising adoption of generics because these drugs became widely available through emerging global generic approvals. Healthcare organizations that seek cost reduction favor generic fondaparinux because this treatment provides cost-effective anticoagulation therapy.
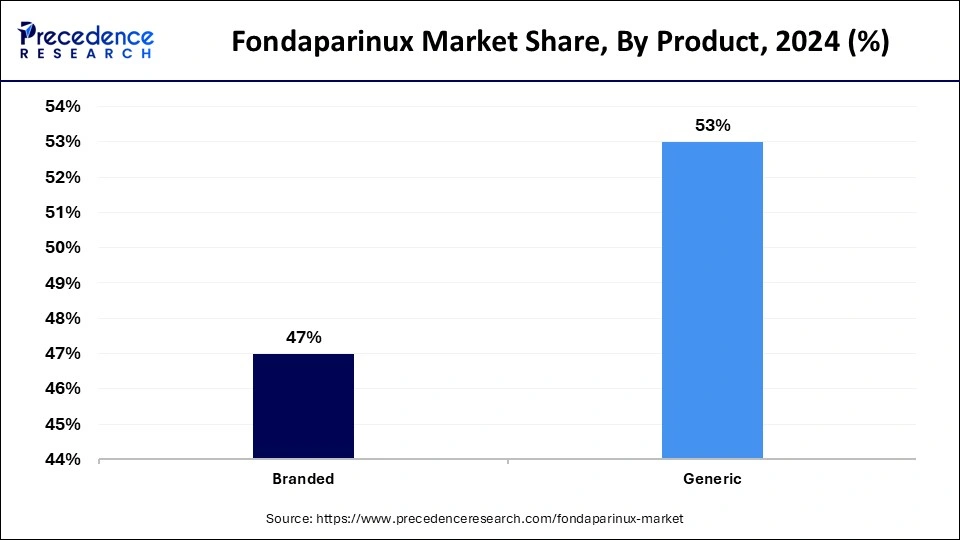
Modern manufacturing technologies help the production process to develop easy methods to reduce operational expenses. Generic utilization receives government support through improved health systems and expanded patient access while providing cost-affordable solutions.
Fondaparinux branded products are expected to grow substantially within the study period of the Fondaparinux market. Branded fondaparinux is preferred for patients and medical providers because of its established expertise and proven clinical capabilities. People tend to trust medicament-bearing brands because the drugs they contain undergo thorough clinical testing to validate their safety, potency, and effectiveness.
The demand for branded fondaparinux exists because patients recognize its superior quality benefits, its continued clinical validation, and its established strong brand position. The segment expands because consumers continually seek trusted, high-quality pharmaceutical products such as these when treating specific high-risk medical conditions.
The retail pharmacies segment accounted for the largest share of the fondaparinux market. The market growth is due to rising cardiovascular disease cases that boost the need for anticoagulants, particularly fondaparinux. The rise in elderly patients and their susceptibility to thromboembolic conditions creates a rapid need for anticoagulant medications. Healthcare expenses continue increasing which expands the market segment because patients need more accessible treatment options.
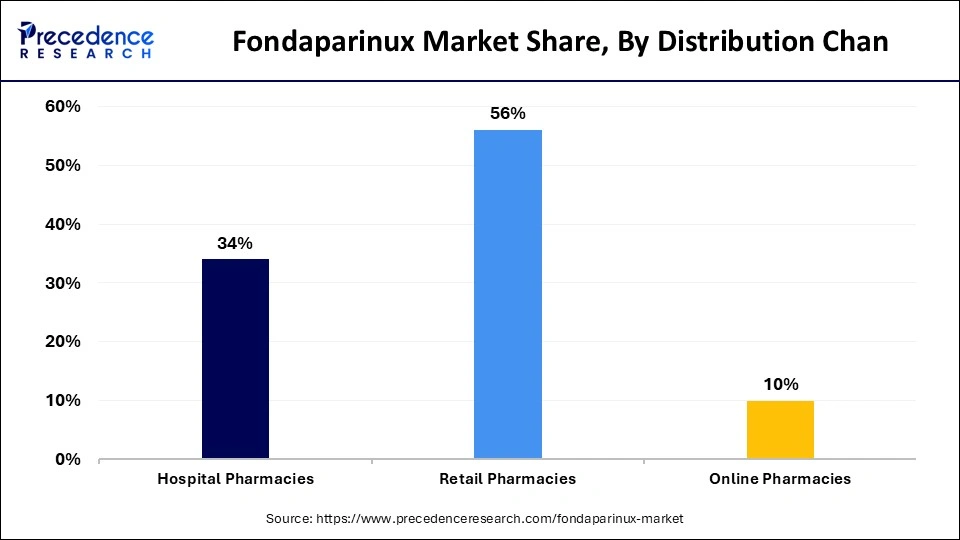
Retail pharmacies take advantage of digital innovations and e-commerce growth that allow consumers better access and more convenient purchase of medications. The segment of retail pharmacies remains the market leader because consumers increasingly depend on them for obtaining essential drugs.
The online pharmacies segment is anticipated to witness significant growth in the Fondaparinux market over the studied period. The growing popularity of e-platforms for medicine acquisition represents a fundamental market force within this sector. Through their digital platform, people can easily obtain fondaparinux medication while remaining in their homes because online pharmacies grant improved accessibility. This segment has seen a boost from telemedicine developments because patients have access to virtual healthcare provider visits, electronic prescription methods, and online medication orders.
Online pharmacies are gaining popularity because patients value home delivery convenience and the growing popularity of digital healthcare solutions. The rising utilization of digital health solutions by consumers will enhance the significance of online pharmacies for distributing fondaparinux in the upcoming year.

By Type
By Product
By Distribution Channel
By Region
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client