February 2025
The global molecular diagnostics market size is estimated at USD 45.11 billion in 2025 and is forecasted to worth around USD 63.86 billion by 2034, accelerating at a CAGR of 3.87% from 2025 to 2034. The North America molecular diagnostics market size crossed USD 17.47 billion in 2024 and is expanding at a CAGR of 3.90% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global molecular diagnostics market size was valued at USD 43.68 billion in 2024 and it is expected to hit around USD 63.86 billion by 2034 with a registered CAGR of 3.87% from 2025 to 2034.
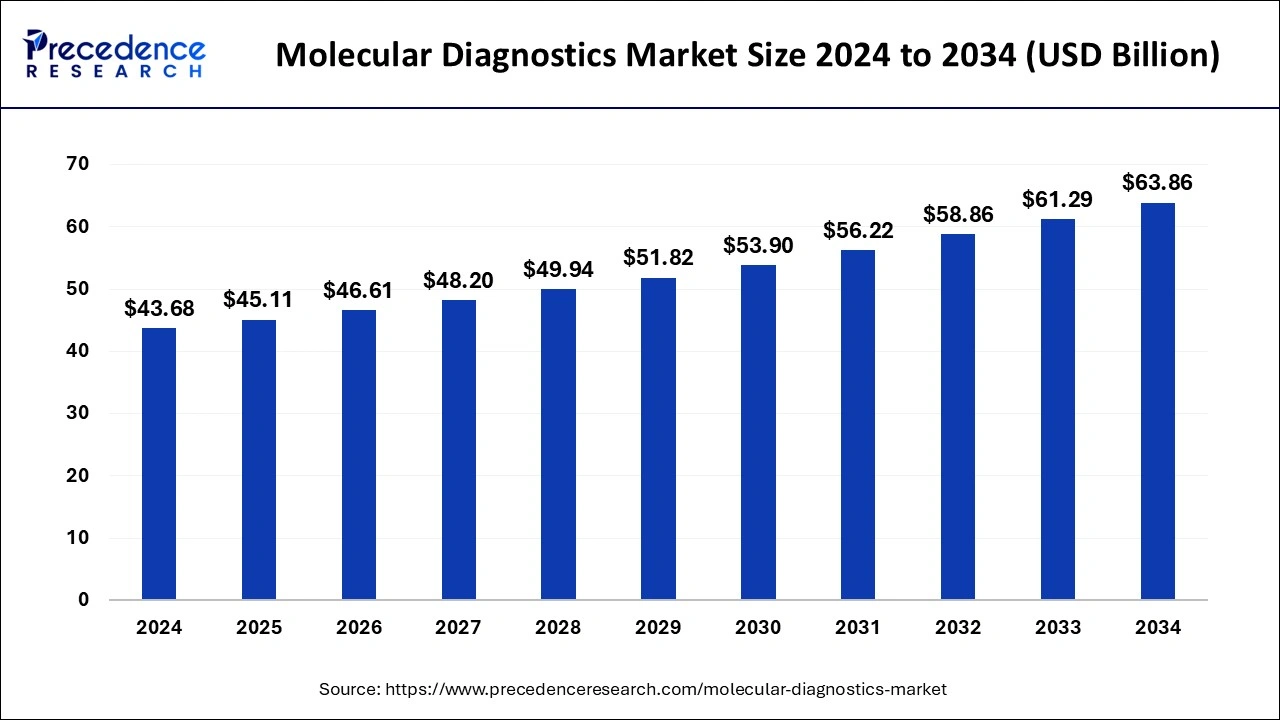
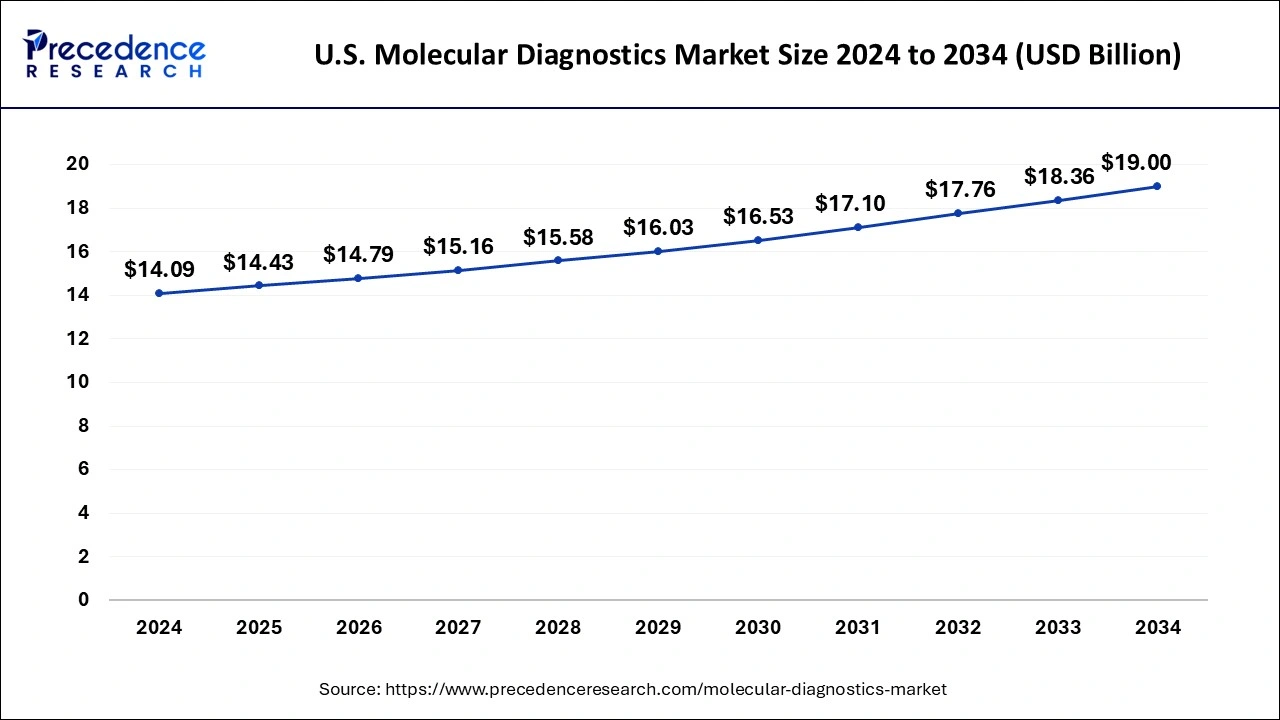
The U.S. molecular diagnostics market size was valued at USD 14.09 billion in 2024 and is expected to reach around USD 19.00 billion by 2034, growing at a CAGR of 3.03% from 2025 to 2034.
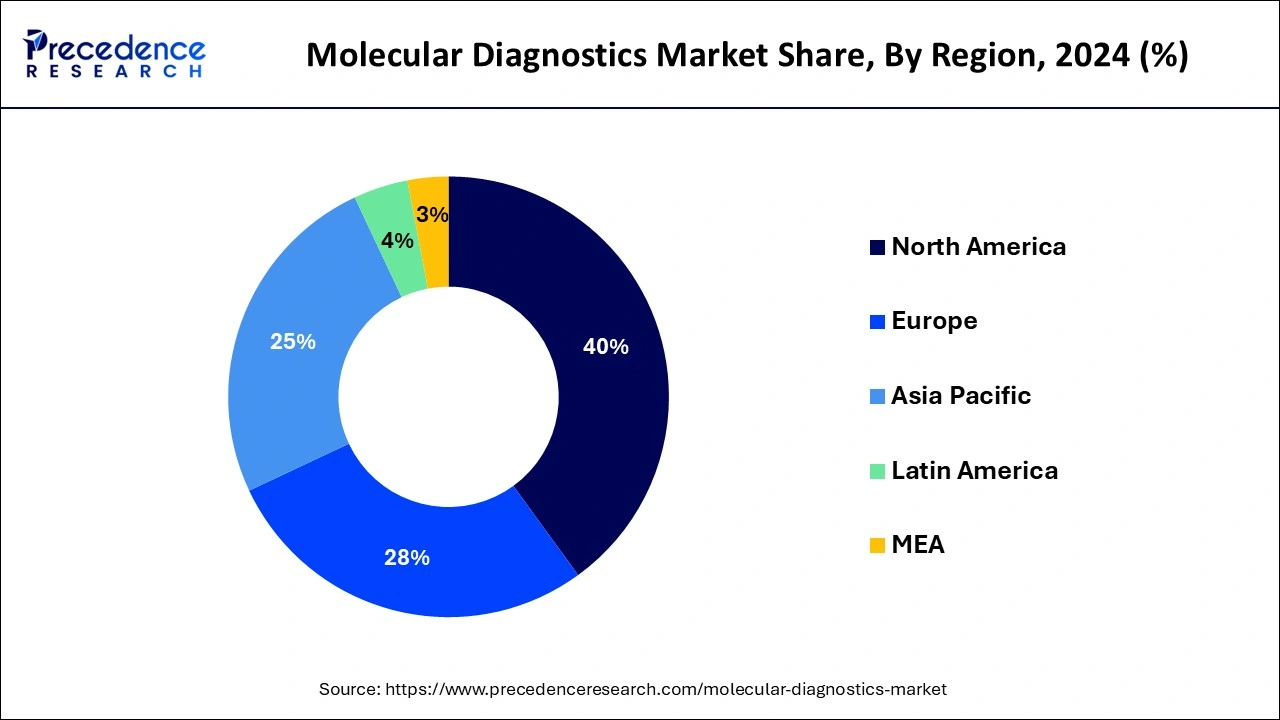
North America holds the dominating share in the molecular diagnostics market. The region has a relatively high prevalence of chronic diseases such as cancer, cardiovascular diseases, and infectious diseases. Molecular diagnostics play a crucial role in the early detection, diagnosis, and monitoring of these conditions, driving the market's growth.
North America is a hub for technological innovation and research in the field of molecular diagnostics. The region is home to many leading biotechnology and diagnostic companies that drive the development and commercialization of cutting-edge molecular diagnostic technologies. North America has one of the highest healthcare expenditures globally. The substantial investment in healthcare infrastructure, research, and development facilitates the adoption of advanced diagnostic technologies, including molecular diagnostics.
Europe Market Trends
Europe is the fastest growing market for the molecular diagnostics market with a significant CAGR during the forecast period, driven by the increasing need for personalized treatment, widespread implementation of advanced diagnostic tools, and supportive governmental policies. The growing prevalence of cancer, infectious diseases, and genetic disorders has spurred the adoption of molecular testing technologies. Significant investments in research and development, a well-established healthcare system, and rising awareness among patients and healthcare practitioners further propel this progress. Nations such as Germany and the UK are at the forefront of innovation in precision diagnostics, whereas EU regulatory harmonization is facilitating cross-border market growth.
Germany
Germany plays a pivotal role in Europe’s molecular diagnostics sector, backed by sophisticated healthcare systems and strong investments in biotech innovation. The nation prioritizes early disease identification and preventive healthcare, which boosts the demand for molecular testing. Government initiatives promoting the integration of digital health and precision medicine within clinical environments are also improving adoption rates. Moreover, collaborations between research organizations and diagnostic firms continue to speed up new product development and commercialization.
Asia Pacific Market Trends
Asia Pacific is observed to grow at a considerable growth rate in the upcoming period, driven by increased healthcare spending, a rising incidence of chronic and infectious diseases, and the expansion of diagnostic laboratories. Nations like China, India, and Japan are investing in biotechnology infrastructure and public health screening initiatives. Technological advancements in PCR, NGS, and point-of-care testing are being increasingly utilized in hospitals and diagnostic facilities. Enhanced healthcare accessibility, heightened awareness, and a large aging demographic further stimulate the regional need for accurate, timely diagnostics, positioning Asia Pacific as a crucial market for global expansion strategies.
China
China’s molecular diagnostics market is quickly evolving due to substantial governmental backing, ongoing healthcare reforms, and heightened public health awareness. The demand for early disease detection tools, especially during and following the COVID-19 pandemic, has resulted in a spike in PCR and NGS-based testing. Strategic collaborations between local biotech companies and international diagnostics firms have strengthened the innovation pipeline, while a burgeoning middle class continues to drive the demand for high-quality diagnostic services.
The increase in the geriatric population globally and an increase in the attracting numerous diseases has led to a growth in the market. Diseases like cancer, cardiovascular diseases, neurological disorders, diabetes and obesity which could be linked to the rising geriatric population is driving the growth of the market. An increase in the incidence of infectious diseases is expected to propel the market growth.
Increase in various other diseases like HIV, HPV and STI shall help in expanding the market. There is an increase in the initiatives taken by the major market players in order to improve the access to various cost-effective resources that they offer. Infections are anticipated to drive the molecular diagnostics market growth during the forecast period till the year 2033. There is an acute and effective result generation in the molecular diagnostic tests and it has indispensable application in many disease diagnostics. These molecular tests or associated with high prices.
Factors like the technological advancements, rising elderly population and the increase in demand for genetic testing is creating great opportunities for the growth of this market. Also, an increase for self-testing diagnostic and the awareness among the patient about these faster diagnostics is creating a great growth.
| Report Coverage | Details |
| Market Size in 2025 | USD 45.11 Billion |
| Market Size by 2034 | USD 63.86 Billion |
| Growth Rate from 2025 to 2034 | CAGR of 3.87% |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product, Test Location, Technology, Application, End User, and Geography |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Rising requirement for cancer diagnostics
According to the National Institutes of Health's projection, there will be approximately 1,958,310 cancer cases in the United States in 2023. This number of rising cases of cancer brings demand for early diagnosis of cancer across the globe. Molecular diagnostics plays a crucial role in the early detection of cancer. Molecular diagnostics identify cancer-related biomarkers at an early stage, healthcare providers can diagnose cancer before symptoms arise, leading to earlier treatment initiation. Cancer continues to be a leading cause of morbidity and mortality across the globe, as the cancer cases increase, the demand for accurate and precise diagnostic method also grows. This element is observed to pose a driver for the market.
Development of precision medicine
Precision medicine focuses on tailoring medical treatments and interventions to individual patients based on their unique genetic makeup, lifestyle, and environmental factors. Molecular diagnostics play a crucial role in this approach by enabling the identification of specific biomarkers, genetic mutations, and gene expression patterns that can guide treatment decisions for each patient. Molecular diagnostics have become an essential component of the drug development process. Companion diagnostics are tests used to identify patients who are most likely to respond positively to a particular treatment. By identifying suitable candidates for specific therapies, companion diagnostics help pharmaceutical companies improve the success rates of clinical trials and streamline the drug development process. Thereby, drives the growth of the market.
Presence of alternative diagnostic methods
Alternative diagnostic methods, such as traditional laboratory tests or imaging techniques, may already be established and widely accepted in the medical community. When molecular diagnostics compete with these existing methods, it can be challenging to gain market share and acceptance, especially if the alternatives are perceived as more cost-effective or easier to implement. Molecular diagnostics often involve sophisticated technologies and specialized equipment, making them more expensive than some conventional diagnostic methods. This cost factor can hinder their adoption, especially in resource-limited settings where healthcare facilities may not have the financial means to invest in expensive molecular diagnostic infrastructure. Thus, the presence of alternative testing methods in the market creates a restraint for the market.
Stringent regulatory policies
Stringent regulatory requirements act as barriers to entry for new entrants in the molecular diagnostics market. As a consequence, established companies with already approved products may have a competitive advantage, making it difficult for newcomers to gain a foothold. Regulatory agencies often work closely with healthcare payers to determine the coverage and reimbursement of molecular diagnostic tests. Uncertain or restrictive reimbursement policies can create financial uncertainties for companies, affecting their willingness to invest in research and development, market expansion, or product improvement. Such policies or guidelines are observed to hamper the market’s expansion.
Rising focus on less-invasive testing methods
Less invasive testing methods can enable earlier detection and continuous monitoring of diseases, leading to better treatment outcomes and overall patient management. Patients often prefer non-invasive or minimally invasive procedures as they are less painful, have shorter recovery times, and reduce the risk of complications. The aging population is more prone to chronic diseases that require frequent monitoring, making less invasive testing methods particularly beneficial for this demographic. Molecular diagnostics has enabled the development of blood tests that can detect specific genetic, or protein markers associated with various diseases. These tests can provide valuable information without the need for invasive procedures, making them more appealing to both patients and healthcare providers. Thus, the rising demand for less invasive testing methods creates significant opportunities for the molecular diagnostics market.
Data safety challenges
Molecular diagnostics heavily rely on the analysis and sharing of sensitive genomic information, data privacy and security becomes critical issues. The rise in cyberattack and data breaches to personal health information could deter patients and healthcare providers from embracing molecular diagnostics technologies. Concerns about how companies handle, or store genetic data might also deter users, patients or consumers from adopting such services. All these factors associated with data safety concerns create a significant challenge for the market.
The reagent segment had the largest market share until 2024. The revenue share of the reagents segment is expected to maintain its dominance in the coming years. The segment is expected to grow during the forecast period as it is widely adopted in many research and clinical settings. Standard reagents are effective in achieving efficient and extremely accurate results.
Due to the standardization of these results, improved efficiency and the cost-effective nature the segment is expected to support the growth of the market during the forecast period. In February 2022 Roche Diagnostics extended the COVID test portfolio to new cobas 5800 system in the countries that accept the CE mark approval.
On the basis of the test locations, the central laboratories had the largest market share in the recent years. In the future the central laboratory segment is expected to grow as there are high procedure volumes for COVID testing in these laboratories. An increasing number of initiatives that are taken by the government to provide different services like reimbursement for these diagnostics test is another factor that is helping in the growth of this market. There is a growing demand in the development of molecular diagnostic platforms which can also be used in POC settings. Many major market players are designing the molecular diagnostic platforms. The growing development of this platform will offer quick POC results which are expected to provide a boost to the market.
Till the year 2024, the infectious diseases segment accounted for the largest revenue share in the molecular diagnostics market. Due to the use of the tests for the diagnosis of COVID-19 the infectious diseases segment dominated the market. In the coming years the oncology segment is expected to drive the market.
The oncology segment is expected to have a high CAGR during the forecast period. About 19.3 million cancer cases were diagnosed across the globe in the year 2020 in order to provide better access and the improvement in the oncology molecular diagnostics the British in vitro diagnostics association along with NHS England developed a commissioning framework for molecular diagnostics in oncology.
The use of PCR technology to detect various infectious diseases or genetic diseases is expected to drive market growth. The polymerase chain reaction segment had the largest revenue till the year 223 due to its use in the detection of COVID-19 and other infectious diseases in the recent years. DNA sequencing processes and technologies are integrally linked to novel drug development, drug discovery and personalized medicine.
By Product
By Test Location
By Technology
By Application
By End User
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
February 2025
December 2024
February 2025
January 2025