April 2025
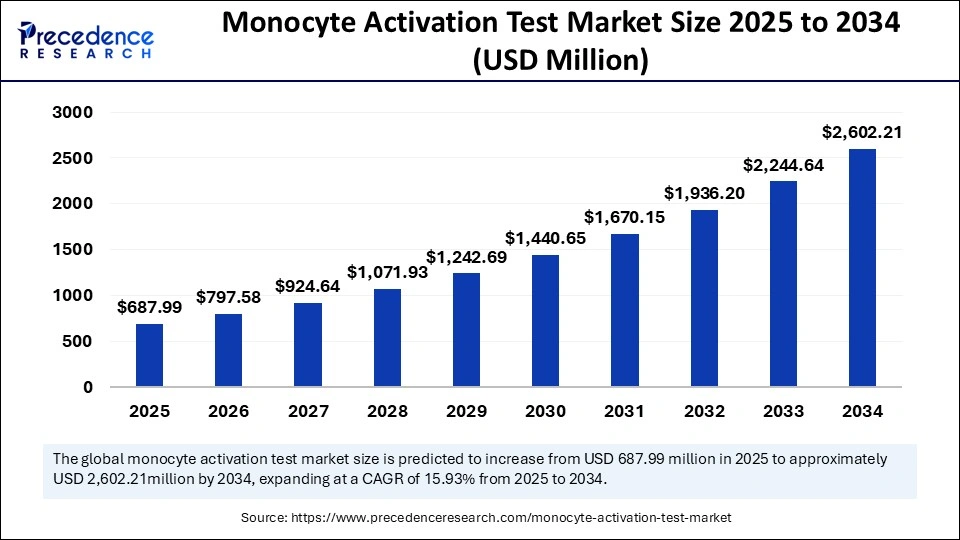
The global monocyte activation test market size is calculated at USD 687.99 million in 2025 and is forecasted to reach around USD 2,602.21 million by 2034, accelerating at a CAGR of 15.93% from 2025 to 2034. The North America market size surpassed USD 219.58 million in 2024 and is expanding at a CAGR of 16.09% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global monocyte activation test market size was accounted for USD 593.45 million in 2024 and is predicted to increase from USD 687.99 million in 2025 to approximately USD 2,602.21 million by 2034, expanding at a CAGR of 15.93% from 2025 to 2034. Growing awareness regarding ethical testing methods is the key factor driving market growth. Also, innovations in monocyte activation test (MAT) technology, coupled with the collaborations between market players, can fuel market growth soon.
Artificial Intelligence plays a substantial role in the monocyte activation test market by improving accuracy, efficiency, and data analysis, contributing to more efficient and safer quality control and drug development processes. Furthermore, AI-powered data analysis techniques help in the effective interpretation of test results, detecting patterns, and facilitating assay parameters to improve overall performance. This enables a more detailed understanding of the test data, which leads to precise and reliable results.
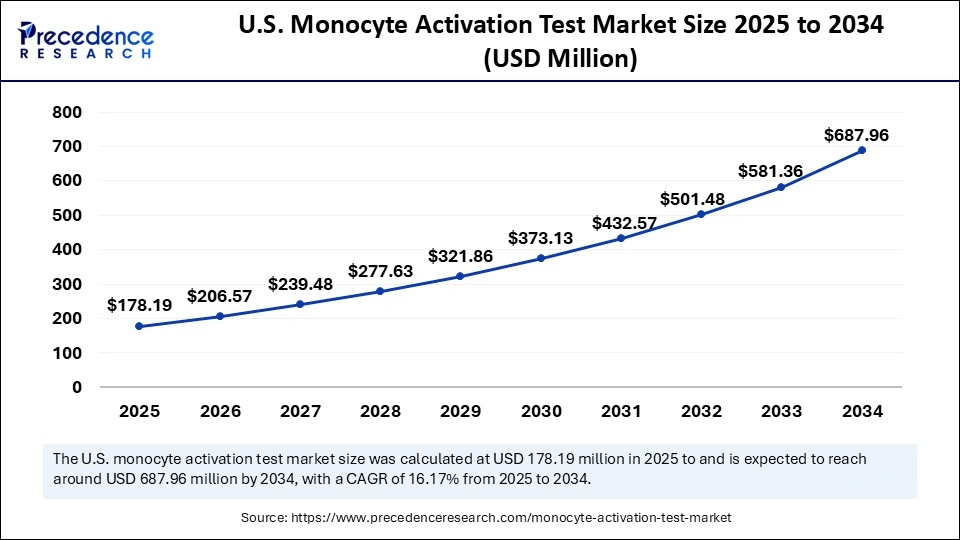
The U.S. monocyte activation test market size was evaluated at USD 153.70 million in 2024 and is projected to be worth around USD 687.96 million by 2034, growing at a CAGR of 16.17% from 2025 to 2034.
North America held the largest monocyte activation test market share in 2024. The dominance of the region can be attributed to the strong presence of well-established medical devices and pharmaceutical industries. Moreover, the region's emphasis on patient safety and quality control has positively impacted regional growth. The region is increasingly adopting innovative diagnostic technologies to extend its market reach.
In North America, the U.S. led the market. The dominance of the country is owing to the country's strong biotechnology and pharmaceutical sectors, along with the stringent regulatory standards, which have fueled the demand for efficient pyogen testing methods such as MAT.
Asia Pacific is expected to show significant growth over the forecast period. The growth of the region can be credited to the region's escalating biotechnology and pharmaceutical industries. Furthermore, countries such as China, Japan, and India are at the forefront of adopting this process because of rising regulatory demands for humane pyrogen testing methods.
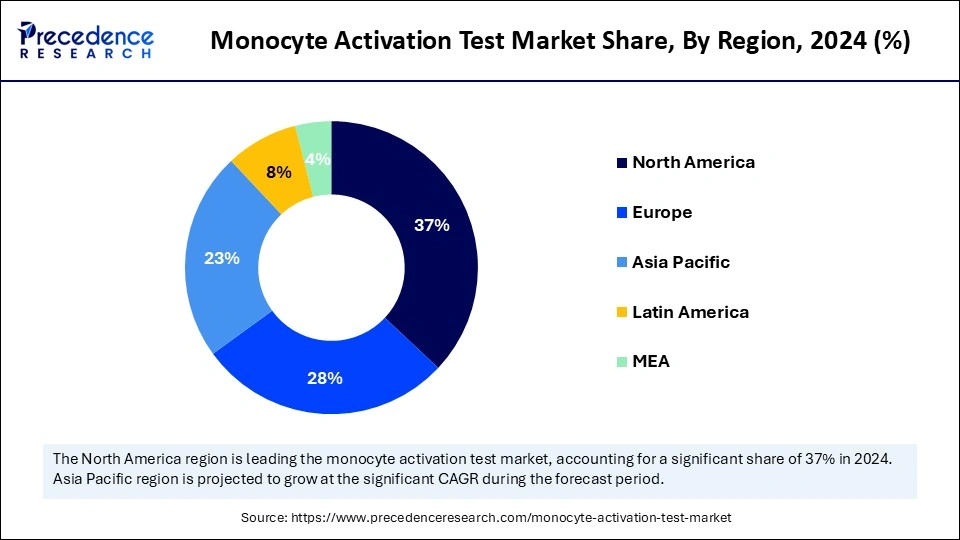
In Asia Pacific, China led the monocyte activation test market in 2024. The dominance of the country is due to the ongoing adoption of MAT by the pharmaceutical sector, boosted by the need for efficient and reliable testing methods. Regulatory bodies are identifying MAT as a crucial option to traditional testing, facilitating its integration into industry practices.
The monocyte activation test market consists of the in vitro assay utilized to identify and quantify pyrogens in medical devices, pharmaceuticals, and other medical products by measuring the human monocyte activation and the release of pro-inflammatory cytokines. It is a human-specific, in vitro option to the conventional rabbit pyogen test, which is being used by government agencies such as the European Pharmacopoeia.
| Report Coverage | Details |
| Market Size by 2034 | USD 2,602.21 Million |
| Market Size in 2025 | USD 687.99 Million |
| Market Size in 2024 | USD 593.45 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 15.93% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product, Source, Application, End Use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Rising incidence of autoimmune diseases
The growing incidence of autoimmune disorders like lupus, rheumatoid arthritis, and multiple sclerosis has substantially raised the demand for monocyte activation tests. In addition, these conditions showcase the subtle interactions between the bodily tissues and the immune system, requiring enhanced diagnostic technologies for trustworthy surveillance and identification.
Standardization of protocols
Implementing monocyte activation test market services in a daily quality control landscape needs the establishment of standardized protocols for cell handling, sample preparation, and assay execution. Moreover, another major hurdle for MAT is navigating the regulatory approvals and obtaining clinical validation. The regulatory regulations can shift between countries, complicating the whole process for organizations looking to market their MAT products in other countries.
Increasing need for individualized care
The increasing shift toward individualized care is creating lucrative opportunities in the monocyte activation test market. MAT plays a crucial role in this scenario by analyzing how a patient's immune system reacts to certain medications, which facilitates the prediction of worst-case events and the treatment strategies. Furthermore, high-throughput screening, automation developments, and flow cytometry are enhancing MAT precision and efficiency.
The MAT kits segment dominated the monocyte activation test market in 2024. The dominance of the segment can be attributed to the increasing use of MAT in pharmaceutical and medical device testing, because of growing regulatory pressure for more efficient products. The MAT is an important segment of a much broader market for kits and reagents, especially in the context of pyrogenicity testing for medical device applications. Hence, MAT is becoming the crucial standard for endotoxin testing.
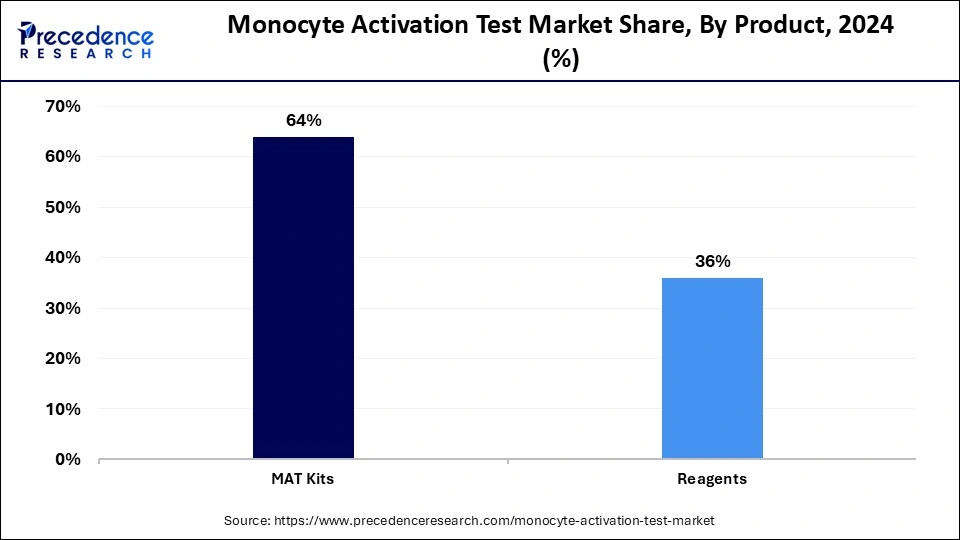
The reagent segment is expected to grow at the fastest rate over the forecast period. The growth of the segment can be credited to the increasing focus on the development and manufacturing of biologics.MAT reagents are essential for detecting pyrogens to ensure product safety. Also, MAT is gaining traction as a reliable in vitro option to conventional methods such as the rabbit pyogen test, especially for identifying non-endotoxin pyrogens.
The PBMC-based segment held the largest monocyte activation test market share in 2024. The dominance of the segment can be linked to the growing demand for more precise and human-relevant testing methods, along with the requirement for safer pharmaceutical products. Additionally, PBMC-based MAT provides a more physiologically relevant model by using human cells, which are more responsive to pyrogens. This is especially important in the testing of vaccines and biologics.
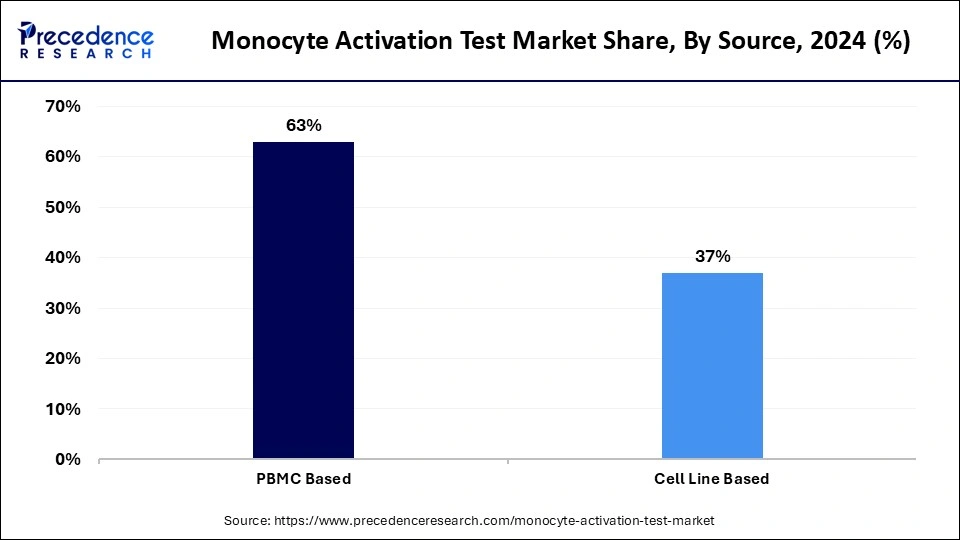
The cell line-based segment is anticipated to show the fastest growth over the forecast period. The growth of the segment can be driven by increasing demand for more standardized and reproducible testing methods. Moreover, this segment provides consistency in results, as it utilizes immortalized cell lines. However, some studies show that MM6-based MAT kits have an inability to identify non-endotoxin pyrogens.
The drug development segment led the monocyte activation test market in 2024. The dominance of the segment is owing to the growing need for safer and more effective pharmaceuticals. MAT is important in identifying pyrogenic contaminants in drugs, which can lead to harmful reactions in patients. Furthermore, the pharmaceutical industry extensively depends on these tests during drug development to optimize overall product safety.
The vaccine development segment is estimated to show the fastest growth over the forecast period. The growth of the segment is due to the rising regulatory emphasis on non-animal testing methods. MAT plays a key role in ensuring safety by identifying pyrogens that lead to harmful reactions like fever. MAT, utilizing human immune cells, provides a more precise and ethical solution to animal-based pyrogen testing.
In 2024, the pharmaceutical industry segment led the monocyte activation test market by holding the largest market share. The dominance of the segment can be credited to the important role of these companies in drug development and safety testing. MAT is crucial in identifying pyrogens to ensure that pharmaceutical products are free from impurities that lead to adverse immune reactions. Hence, MAT is becoming the preferred choice for pyrogen testing in the pharmaceutical industry.
The biotechnology industry segment is expected to grow at the fastest rate over the projected period. The dominance of the segment can be linked to the increasing awareness of health issues and raised government funding for research and innovations in gene editing. Also, a major driver for MAT adoption is the growing regulatory demand for human-relevant and reliable testing methods. MAT helps to fulfill these requirements by providing faster results.

By Product
By Source
By Application
By End Use
By Region
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
April 2025
January 2025
October 2024
September 2024