January 2025
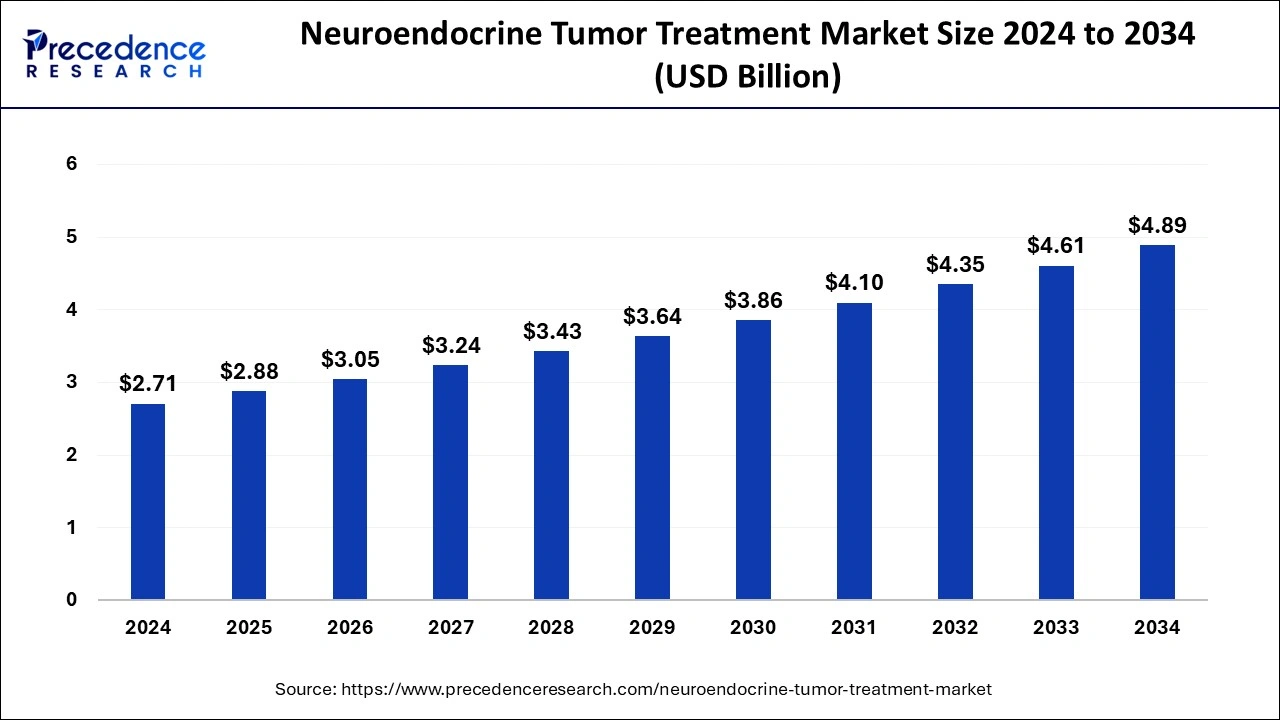
The global neuroendocrine tumor treatment market size is calculated at USD 2.88 billion in 2025 and is forecasted to reach around USD 4.89 billion by 2034, accelerating at a notable CAGR of 6.09% from 2025 to 2034. The North America market size surpassed USD 1.14 billion in 2024 and is expanding at a CAGR of 6.19% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global neuroendocrine tumor treatment market size accounted for USD 2.71 billion in 2024 and is expected to exceed around USD 4.89 billion by 2034, growing at a CAGR of 6.09% from 2025 to 2034. Growing awareness of neuroendocrine tumors and the importance of early detection are driving the growth of the neuroendocrine tumor treatment market. The growing demand for personalized medicines is contributing to the market expansions. Moreover, growing research and development activities are likely to boost the market expansion in the forecast period.
The integration of artificial intelligence is significantly transforming the neuroendocrine tumor treatment market by enhancing diagnostic accuracy, personalized medicine plans tailored to the patient, and streamlined clinical trials. AI is enabled to identify suitable candidates for clinical trials, as well as optimize trial design and result analysis to improve efficiency. AI-enabled chatbots and VAs help to minimize burdens and allow healthcare professionals to concentrate on critical tasks. The ability of AI to predict treatment response is helping to generate targeted and effective treatment solutions.
AI also helps to identify effective treatment options for certain patients to improve patient outcomes and quality of life, which makes it easier for healthcare professionals. Additionally, AI is able to identify possible risks, which helps with risk management during trials as well as diagnostics. AI plays a crucial role in surgeries and finding rare diseases. The integration of AI is overall shifting the neuroendocrine tumor treatment market toward success by enhancing treatment, diagnosis, and patient outcomes.
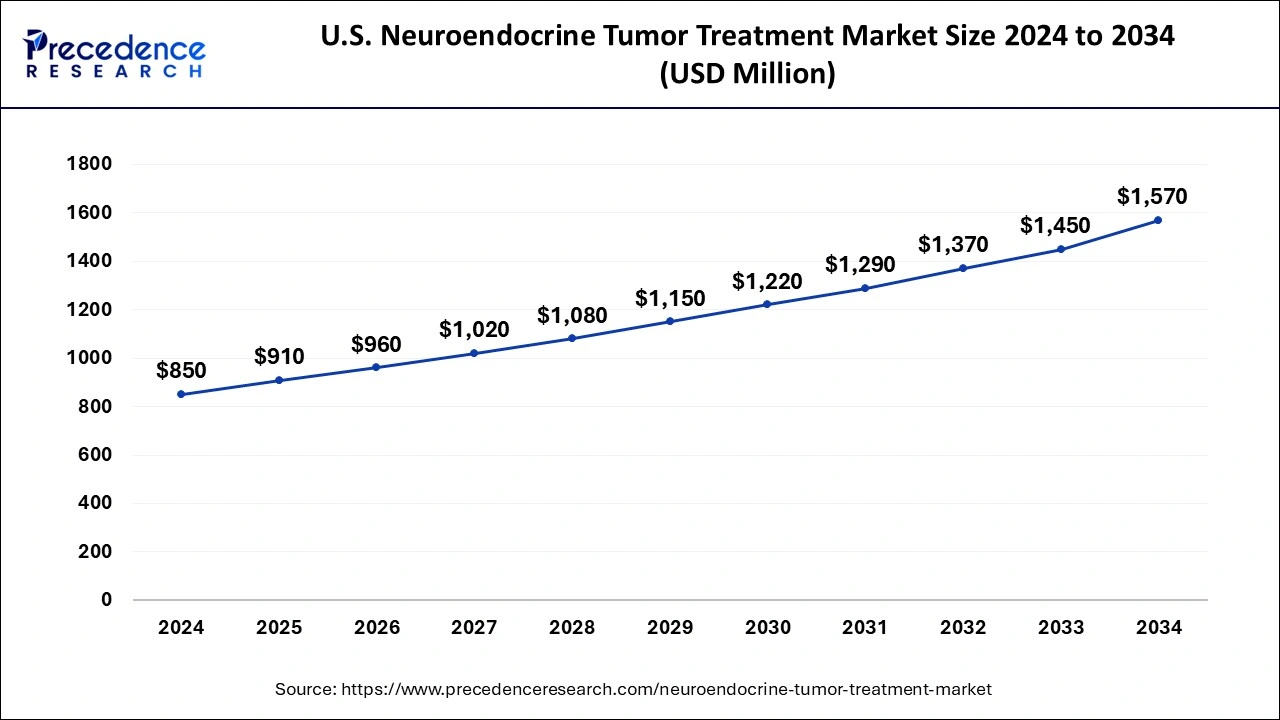
The U.S. neuroendocrine tumor treatment market size was exhibited at USD 850 million in 2024 and is projected to be worth around USD 1,570 million by 2034, growing at a CAGR of 6.32% from 2025 to 2034.
North America dominated the global neuroendocrine tumor treatment market with the largest market share in 2024 for several reasons, including an expanded, well-established healthcare sector, pharmaceutical companies, government investments in research and development firms, and strict regulations. The government of North America has diverted its focus toward research and development of cutting-edge techniques for early detection and diagnostics in oncology due to the rapidly increasing prevalence of neuroendocrine tumor cases in the region.
The advanced healthcare infrastructure and providing access to advanced diagnostic and therapeutic technologies. The United States is leading the regional market due to a stringent regulatory framework that invests in the development of innovative neuroendocrine tumor treatments.
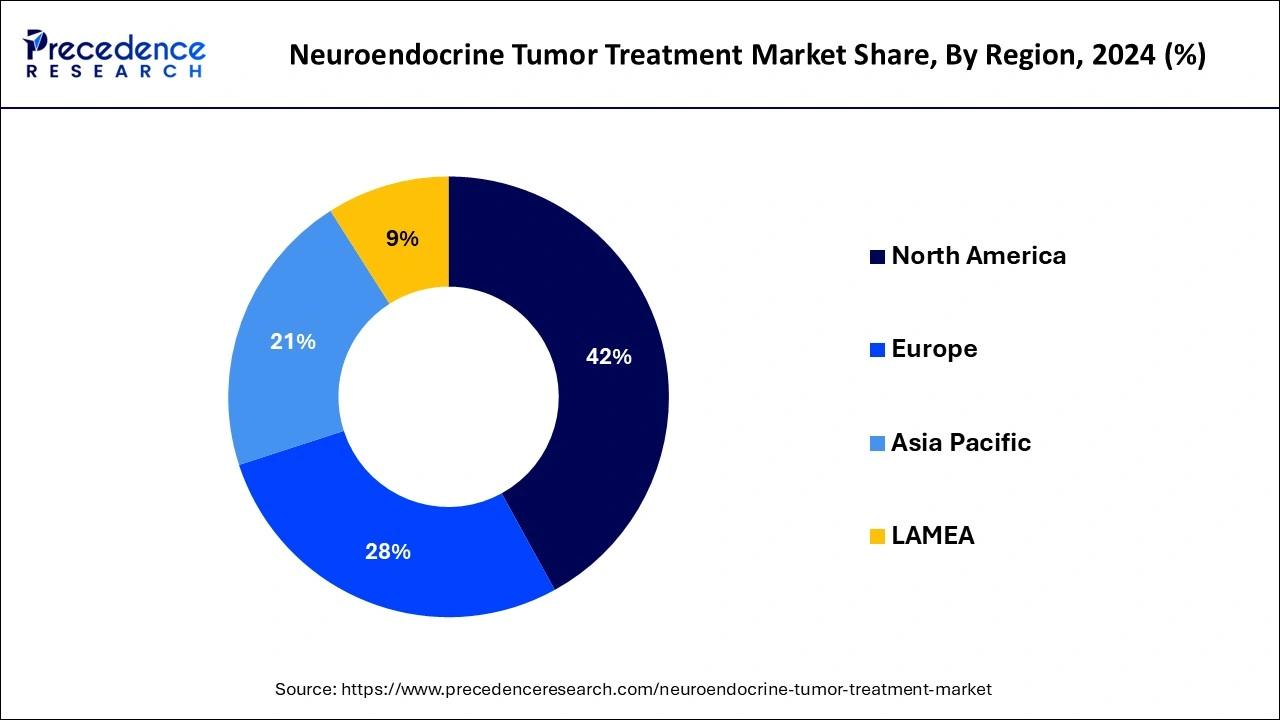
Europe is anticipated to witness significant growth in the neuroendocrine tumor treatment market during the forecast period due to the vast pharmaceutical infrastructure in the region. Government support and funding for research and developments in the pharmaceutical and healthcare sectors are the key factors enhancing market growth in the region. The prevalence of neuroendocrine tumor treatments has rapidly increased in Europe. The presence of well-established healthcare systems in Europe is allowing access to advanced therapies. Additionally, regions are drifting in market success due to the availability of high-quality healthcare professionals.
Regulatory initiatives like the European Medicines Agency (EMA) are supporting the development of novel, innovative treatment options. Moreover, companies are easily launching their novel treatment innovations with the support of a harmonized regulatory environment. The ongoing collaborations between universities, cancer centers, and research institutes are further highlighting innovations. Additionally, partnerships between institutes and organizations are developing and commercializing new NET treatments.
The neuroendocrine tumor (NET) is a rare cancer that occurs due to abnormal growth of cells in the neuroendocrine system. The growing prevalence of neuroendocrine carcinoma and growing awareness of the NETs are the key factors expanding the growth of neuroendocrine tumor treatments. NETs are very common in older populations; also, changing lifestyles and environmental issues are causing incidences of neuroendocrine tumors. The awareness of prevalence and early detection has risen in recent years, which has led to the development of innovative therapy options. Additionally, pharmaceutical companies and governments worldwide have enhanced investments in the research and development sector for modern and effective treatment solutions.
The developments of novel medications to identify various types of carcinoid tumors and target therapies, as well as immunotherapy, are shifting the neuroendocrine tumor treatment market rapidly toward success. Additionally, the development of novel, innovative radioligand therapy is emerging in the market. The adoption of new medications, like imaging techniques, biomarkers, target therapies, and precision medicines, is crucial for improving the market conditions. The growing partnerships of academies and pharmaceutical companies are seeking new ways to improve market expansions by working together in research and clinical trials. Additionally, the market is expected to witness significant growth in the forecast period due to increasing sustainability trends and regulatory support.
| Report Coverage | Details |
| Market Size by 2024 | USD 2.71 Billion |
| Market Size in 2025 | USD 2.88 Billion |
| Market Size in 2034 | USD 4.89 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 6.09% |
| Leading Region | North America |
| Fastest Growing Region | Middle East and Africa |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Type, Product, Site, End-use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Increased prevalence of neuroendocrine tumor diseases
The prevalence of neuroendocrine tumors has increased in recent years due to changing lifestyles and environmental impacts. Growing obesity and physical inactivity are leading to the prevalence of the disease; also, the diseases are common in the aging population. The diagnosis of NET patients is mostly done in stage 4, making it riskier and more challenging. This complexity in neuroendocrine tumors is driving the importance of research and development in the neuroendocrine tumor treatment market. However, advanced diagnostic techniques like computed tomography (CT scans), PET scans, and magnetic resonance imaging (MRI) allow for the early detection of such and more accurate diagnoses for the NETs. Moreover, the adoption of endoscopy provides more improved diagnostic treatments for NETs.
Additionally, increased awareness of neuroendocrine tumors and the importance of early detection are encouraging developments of cutting-edge diagnoses and treatment solutions. The increased prevalence of NETs has led to the adoption of innovative therapies like targeted therapies, immunotherapies, and combination therapies that are fueling the neuroendocrine tumor treatment market expansions. Moreover, the increased reporting of the NET cases is expected to attract more attention from government and regulatory bodies to invest in innovation and development firms that will help the market for further growth.
High cost and complexity
The neuroendocrine tumor treatment market services are expensive, which is pressurizing for the patients. The cost of pharmaceutical manufacturing, as well as the development of new therapies, are impacting overall treatment costs. NETs have complex biology and clinical behavior that is challenging in the development of effective and targeted treatment solutions.
Lack of awareness and regulatory challenges
The awareness of neuroendocrine is very low among health professionals and patients; mostly, patients are taking treatments at stage 4, which leads to an unliveable solution that directly hinders the neuroendocrine tumor treatment market expansion. The stringent regulatory requirements for approvals and high costs associated with clinical trials are hampering the research and development sector's growth for neuroendocrine tumor treatments.
Limited diagnostic accuracy
The lack of specific biomarkers is making it difficult to provide accurate diagnoses for NETs due to limited access to track disease progressions and predictions for required treatment options.
Technology advancements
The developments of advanced treatment methodologies are projected to boost the neuroendocrine tumor treatment market expansion in the forecast period. The adoption of cutting-edge treatments, such as target therapies like somatostatin analogs and tyrosine kinase inhibitors, is enhancing patient outcomes. Similarly, personalized medicines have been popular with target therapies and precision medicine demands. Moreover, technological developments in genomics, as well as molecular biology, are allowing an improved understanding of the biological mechanisms of NETs, making it easier to generate targeted therapies.
Diagnostic technologies like CT scans, PET scans, and MRIs have been improved to provide more accurate and early diagnoses for the neuroendocrine tumor treatment market. The increased prevalence of NETs is driving the importance of early detection and diagnosis, leading to developments as well as options for advanced technologies by healthcare professionals. Government and regulatory initiatives are also contributing to these developments. Additionally, pharmaceutical companies have increased investments in research and development for innovative treatment options and expanded the treatment methodologies for neuroendocrine tumors.
The carcinoid tumors segment captured the biggest neuroendocrine tumor treatment market share in 2024 due to the increased incidence of carcinoid tumors. The segment has witnessed growth due to increased awareness of early diagnosis and demand for improved diagnostic techniques. The availability of advanced treatment options for carcinoid tumors, like chemotherapy, targeted therapies, and somatostatin analogs, is also helping the segment to grow. Ongoing research with vast clinical trials investigating new treatments and combination therapies, as well as a focus on advancing immunotherapies and target therapies, are driving development for carcinoid tumors.
On the other hand, the paraganglioma segment will show notable growth in the neuroendocrine tumor treatment market during the forecast period. Paragangliomas are rare and require specific treatment approaches, including radiation therapy, systemic therapies, and surgery. The increased focus of researchers on enhancing target therapies like tyrosine kinase inhibitors to treat paraganglioma is the key factor driving the growth of this segment. Increased incidence of paraganglioma is driving awareness for earlier diagnosis and treatments and increasing demand for advanced and effective treatment options. Continuous research initiatives to improve treatment solutions for paraganglioma.
The somatostatin analogs (SSAs) segment contributed the highest neuroendocrine tumor treatment market share in 2024 during the forecasted years with its proven record of efficacy and safety. The somatostatin analog has become the first-line treatment option for NETs like carcinoid tumors. SSAs have gained approval for treatments of several NETs, like carcinoid tumors, pancreatic NETs, and gastrointestinal NETs. SSAs are available in a wide range of dosing options, including oral and injectable, which are convenient and comfortable for patients, especially aging populations. The ongoing research for the use of SSAs in lung NETs and thyroid NETs.
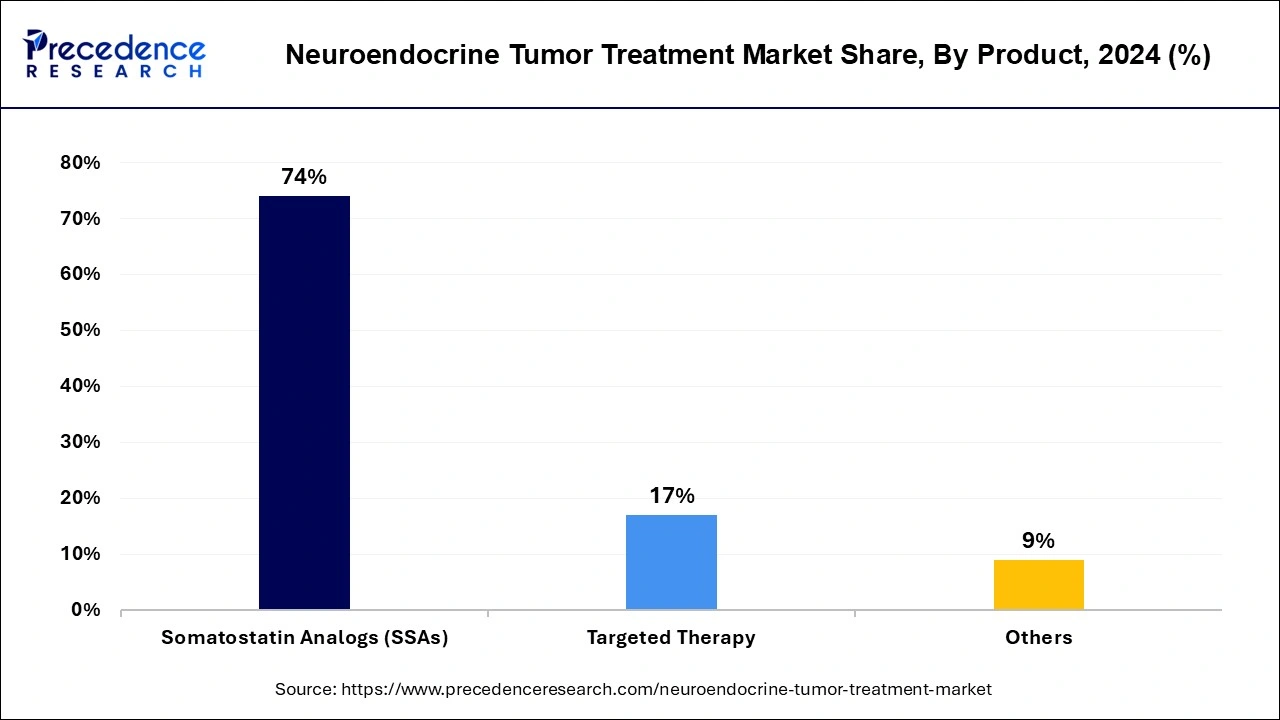
However, the targeted therapy segment is anticipated to witness the fastest growth in the neuroendocrine tumor treatment market during the forecasted years due to its improved effectiveness in the treatment of various NETs, including pancreatic and gastrointestinal NETs. The increased focus on personalized medicines for specific tumors is rapidly expanding the segment expansion. Additionally, the ability of target therapies to deliver enhanced treatment outcomes and reduce side effects is making them preferred by healthcare professionals, with ongoing research and developments for continuous advancements and a growing need for effective treatment options.
The small intestine segment generated the major share in the neuroendocrine tumor treatment market in 2024. Small intestines are the common site for NETs; the major incidence of NETs occurs in small intestines. The need for specialized treatment options for small intestine NETs has increased focus on research and developments of novel, cutting-edge treatment solutions that are responsible for fueling market growth. Additionally, the availability of advanced treatment options like somatostatin analogs, targeted therapies, and chemotherapy are contributing to segment expansions. The ongoing developments of peptide receptor radionuclide therapy for small intestine NETs.
On the other hand, the stomach segment is expected to witness significant growth in the neuroendocrine tumor treatment market over the forecast period due to increased gastric NET incidence. The increased incidence of gastric NETs is driving demand for novel, cutting-edge treatment options like endoscopic resection, surgery, and somatostatin analogs. The increased awareness of stomach-related NETs among healthcare professionals and patients is fueling the segment expansions. Additionally, increased investments in developing new treatment options for gastric NETs.
The hospitals segment accounted for the largest share of the neuroendocrine tumor treatment market in 2024. The segment growth is driven by the availability of well-established treatment care in settings for NET patients in the hospitals. Increased incidence of NETs has increased hospitalization; also, the ability to provide a large volume of NET treatments is driving the making of hospitals dominating the market. Hospitals provide multidisciplinary care teams for oncology, radiology, surgery, and endocrinology, which monitor and provide management for the NETs. Access to advanced diagnostic technologies like CT scans and PET makes hospitals prior to getting treatment forms. Moreover, the availability of cutting-edge treatment technologies like peptide receptor radionuclide therapy (PRRT) and stereotactic body radiation therapy (SBRT) are also contributing to the segment's growth. Growing government and non-government organizations' focus and funding to the hospitals are making easy access to advanced therapies and rising patient outcomes.
However, the clinics segment is expected to show the fastest growth in the neuroendocrine tumor treatment market during the predicted timeframe due to increased demands for specialized care. The segment growth is also driven by the providing of multidisciplinary services that bring together professionals from different fields of oncology, surgery, and endocrinology. The rising need for personalized and patient-centered care is rapidly shifting preference toward clinics. The convenient locations and access for advanced and quick treatments are projected to enhance patient preference for the clinics.

By Type
By Product
By Site
By End-use
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
January 2025
February 2025
February 2025
January 2025