April 2025
Leading companies in the in vivo CRO market are IQVIA, ICON plc, Crown Bioscience, Pharmaceutical Product Development, Inc., TFS HealthScience, Charles River Laboratories International, Inc., Laboratory Corporation of America, Syneos Health, Parexel International Corporation, WuXi AppTec, Inc, Clinipace and others.
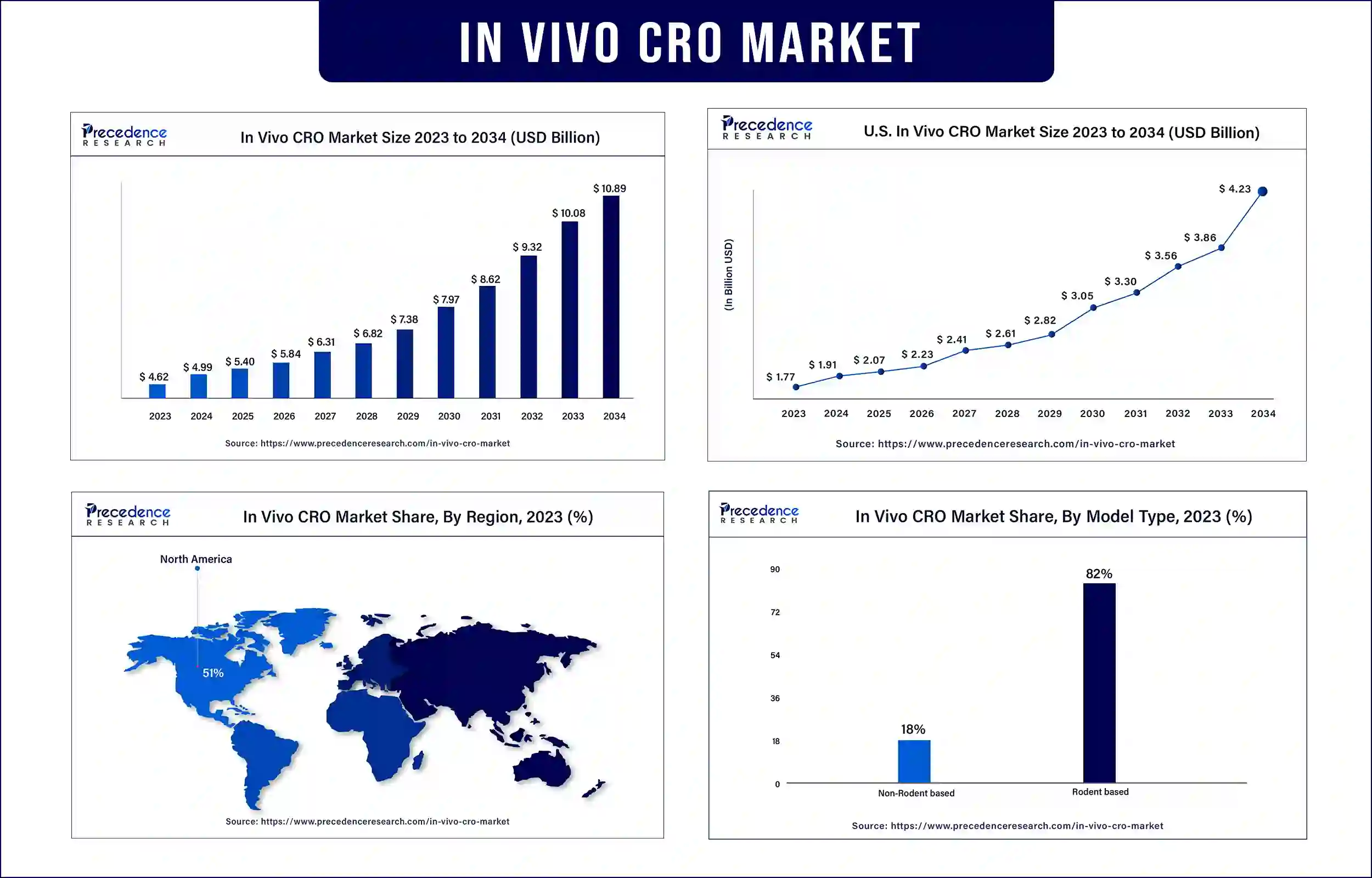
The global in vivo CRO market was exhibited at USD 4.62 billion in 2023 and is anticipated to touch around USD 10.08 billion by 2033, poised to grow at a CAGR of 8.11% during the forecast period. The rising trend of outsourcing drugs is driving the growth of the in vivo CRO market.
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/4783
The in vivo CRO, also referred to as the contract research organization, is the independent body or the research center that helps the pharmaceutical, biotech laboratories, and various government research centers in clinical or medical studies to develop new drugs or treatment procedures for complex disease conditions. The in vivo CRO involves various types of clinical research and services, providing clinical, scientific, and business continuity for the sponsor of clinical trials. CROs are involved in various services, from pre-clinical research to post-marketing surveillance. They provide more accurate insights into drug development with their efficient research strategies. The rising number of clinical research results in the adoption of outsourcing methods, thereby boosting the growth of the in vivo CRO market.
The increasing global population and the increasing prevalence of chronic diseases boost the demand for efficient medication and treatment procedures, significantly increasing the number of clinical research or trials for drug development.
The rising burden of drug development on pharmaceutical companies, biopharmaceutical laboratories, government research institutes, and others is increasing the number of outsourcings of clinical research and medical studies for drug discovery, which drives the growth of the market.
The rising investment in the development of independent research centers for drug development and government initiatives for the development of effective healthcare solutions and drug discovery are boosting the number of contract research organizations that contributing to the expansion of the market.
The in vivo CRO accelerates the drug delivery process
CROs play an important role in drug discovery and delivery and clinical trials. They streamline the challenges associated with drug discovery, maintain regulatory compliance, and drive efficiency in the overall process. CROs provide a wide network of patients and clinical sites. They reduce the time and cost-consuming process of clinical trials using patient recruitment strategies and data management systems. The evolution of the pharmaceutical industry with CRO assistance helps efficiently manage and accelerate the drug discovery process. CROs reduce the cost and time required in the operation, accelerate the entrance of new drugs into the market, and enable specialized expertise and worldwide reach with increased flexibility and scalability. All these benefits drive the collaboration between CROs and the major pharmaceutical companies, contributing to the expansion of the in vivo CRO market.
On the other hand, although CRO has several benefits, some potential limitations also exist
Maintaining seamless coordination and communication between CROs and pharmaceutical companies is challenging, limiting the expansion of the market. Potential risks and challenges in terms of maintaining quality standards, drug discovery and delivery, and research are restraining the growth of the market. Thus, to mitigate these challenges, the selection of right CRO partner is important, which helps in maintaining all the regularities with the increased quality standards of clinical research and drug discovery.
Recent Innovation in the In Vivo CRO Market by IQVIA
| Company Name | IQVIA |
| Headquarters | Durham, North Carolina, U.S. |
| Development | In June 2024, IQVIA introduced One Home for Sites, an advanced platform that acts as a single dashboard and single sign-on for the major tasks and systems that clinical research requires for all clinical trials it conducts. |
Recent Innovation in the In Vivo CRO Market by Crown Bioscience
| Company Name | Crown Bioscience |
| Headquarters | San Diego, California, U.S. |
| Development | In March 2024, Crown Bioscience, a global contract research organization (CRO), and JSR Life Sciences Company are about to showcase an important contribution to preclinical and translational oncology research at the meeting of the American Association for Cancer Research (AACR) 2024. The companies are about to showcase a broad range of revolutionary techniques in oncology and immuno-oncology drug development and discovery. |
Asia Pacific is expected to be the fastest-growing region in the in vivo CRO market during the forecast period. The rising population in countries such as India and China are anticipated to boost innovations in the healthcare and pharmaceutical sectors in the region. The rising prevalence of chronic diseases, especially among the geriatric population, is driving drug development. Furthermore, the increasing involvement of government and private sectors in developing effective drugs is driving innovations in pharmaceutical and outsourcing companies for drug development.
The rising population is the major factor propelling the demand and innovation in drug discovery. Additionally, the rising burden of clinical research on pharmaceutical organizations is accelerating the adoption of CROs for outsourced research on clinical trials, contributing to the expansion of the in vivo CRO market in the region.
North America held the largest share of the in vivo CRO market in 2023, owing to the presence of the well-developed healthcare and pharmaceutical infrastructure and the availability of skilled professionals. Countries such as the US are heavily investing in drug development process. The region also boasts major players in the pharmaceutical sector, accelerating the growth of the in vivo CRO market in the region.
The Inclusion of automation in CROs and pharmaceutical companies is creating new opportunities in the in vivo CRO market
The demand for contract research organization (CRO) outsourcing has increased by 45% in R&D activities. The increasing demand and the continuous rise in the volume of data cases, changing regulations, and data complexities drive the demand for technologically advanced systems to reduce workload. However, the adoption of modern pharmacovigilance (PV) automation helps in overcoming all these pressures and challenges that arise in the research. Pharmacovigilance (PV) automation offers increased efficiency, scalability, flexibility, and cost-effectiveness.
Automation helps CROs reduce manual and repetitive tasks, minimize the risk of consistent errors, comply with changing regulations, decrease the time and cost required for linear data processing, and streamline PV operations. It allows data streams to connect with different sources, enhancing decision-making and risk assessment. Thus, the adoption of automation increases efficiency and improves consistency and data quality. It enables the higher value of initiatives like benefit-risk assessment and PV analytics.
| Report Attribute | Key Statistics |
| Market Revenue in 2024 | USD 4.99 Billion |
| Market Revenue by 2033 | USD 10.08 Billion |
| CAGR | 8.11% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2023 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Segmentation
By Model Type
By Modality
By Indication
By GLP Type
Buy this Research Report@ https://www.precedenceresearch.com/checkout/4783
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308
April 2025
January 2025
January 2025
January 2025