March 2025
The global clinical data management system market size is calculated at USD 3.46 billion in 2025 and is forecasted to reach around USD 8.90 billion by 2034, accelerating at a CAGR of 11.09% from 2025 to 2034. The North America clinical data management system market size surpassed USD 1.43 billion in 2024 and is expanding at a CAGR of 11.22% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global clinical data management system market was estimated at USD 3.11 billion in 2024 and is predicted to increase from USD 3.46 billion in 2025 to approximately USD 8.90 billion by 2034, expanding at a CAGR of 11.09% from 2025 to 2034. The clinical data management system market is experiencing significant growth due to the increasing demand for efficient and reliable management of clinical trial data.
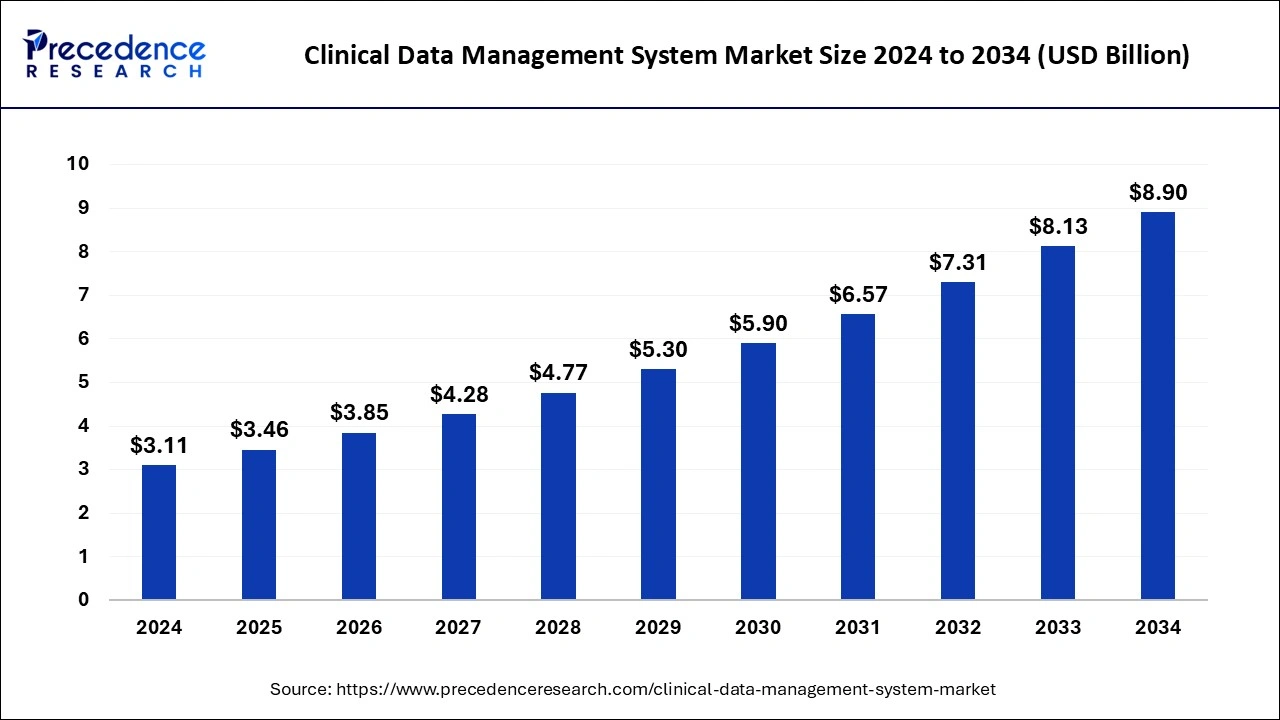
The U.S. clinical data management system market reached USD 1.07 billion in 2024 and is predicted to be worth around USD 3.12 billion by 2034, at a CAGR of 11.30% from 2025 to 2034.
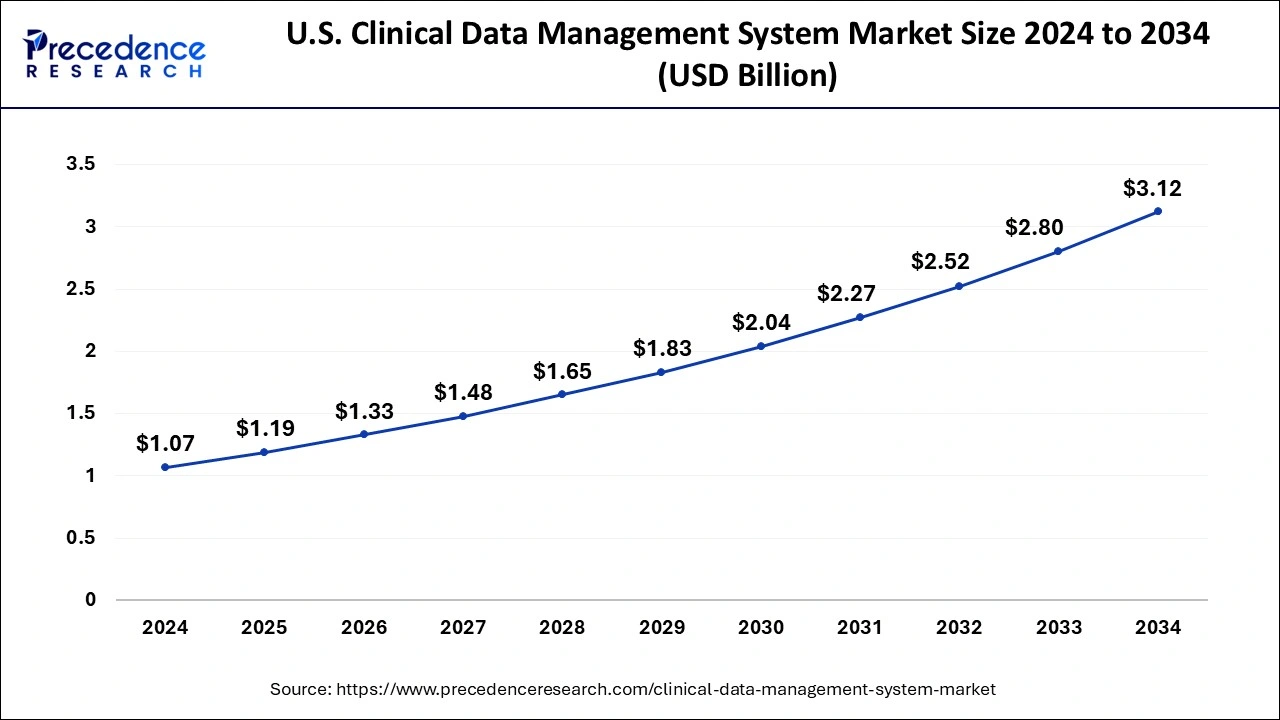
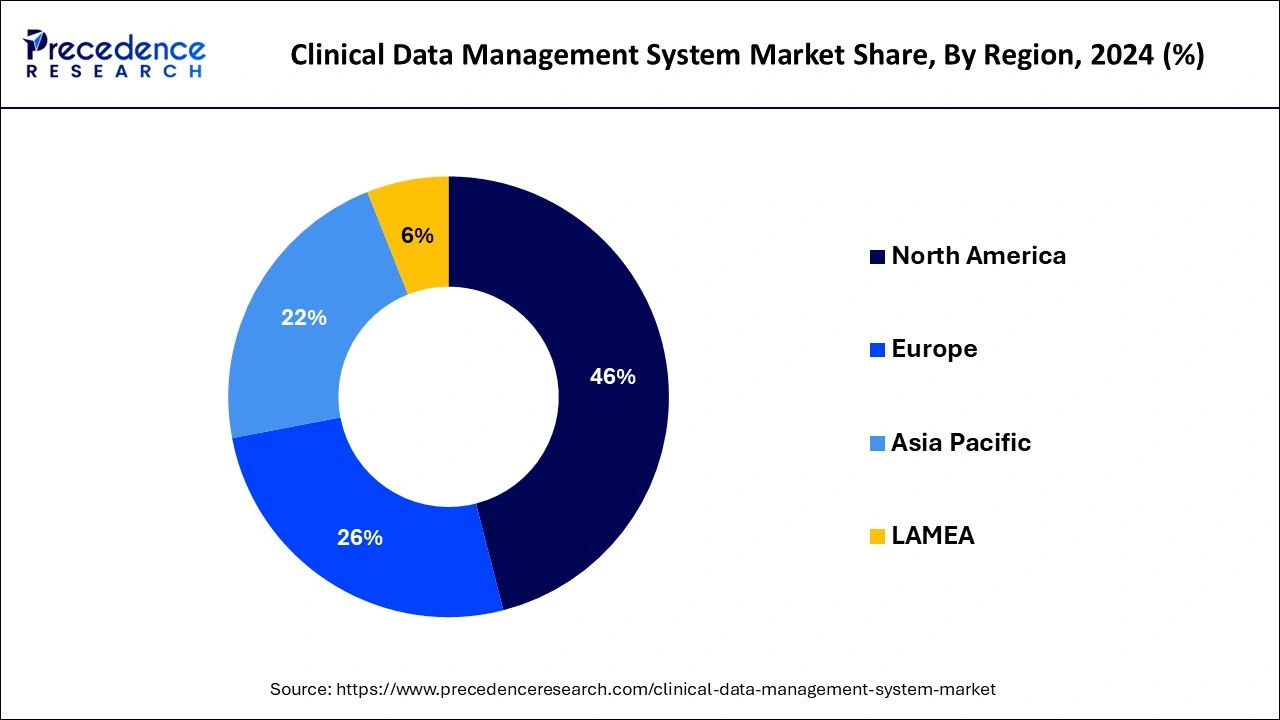
North America was the leading region in the clinical data management system market in 2024, and this trend is expected to continue in the foreseeable future. This dominance can be attributed to the strong presence of renowned pharmaceutical and biopharmaceutical companies and CROs in the region. Additionally, North America's extensive drug development activities have resulted in the widespread adoption of clinical data management systems. Specifically, the United States is witnessing a surge in clinical research studies, leading to a shift from traditional manual data collection and analysis methods to real-time data analysis during clinical research.
Asia Pacific is estimated to attain the fastest CAGR in the upcoming years. This is attributed to the rise in research and development efforts, coupled with a sizable patient population and a growing number of clinical trials, which has contributed to the dominance of the clinical data management system market in the region. Moreover, outsourcing clinical trials to Asian countries, which offer cost-effective research options, is expected to drive market growth in the region further.
India and China stand out as the key players in Asia Pacific, driven by their large populations and robust healthcare industries engaged in significant research and development efforts for novel medications. These dynamics are anticipated to propel the expansion of the clinical data management system market in the region throughout the forecast period.
A clinical data management system (CDMS) is designed to ensure that patient care and treatment data are well-organized, easily accessible, and secure. It helps healthcare professionals stay informed about patient histories, allergies, test results, and other important information. By centralizing this data in a secure location, CDMS facilitates better communication among healthcare providers and improves patient care coordination.
In clinical research, the clinical data management system market plays a crucial role in efficiently managing the collection, organization, and oversight of data generated during clinical trials. It is used throughout the entire lifecycle of a clinical study, from study initiation to data collection, analysis, and reporting. CDMS allows researchers to record and manage various types of clinical trial data, including medical histories, lab results, patient demographics, and adverse events. By ensuring data reliability and compliance with regulatory standards, CDMS promotes collaboration among research teams through a centralized platform for data entry and monitoring.
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 11.09% |
| Market Size in 2025 | USD 3.46 Billion |
| Market Size by 2034 | USD 8.90 Billion |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | By Delivery Mode, and By End-use |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Rising number of clinical trials
The clinical data management system market is experiencing significant growth due to the rising number of global clinical trials. Industries like pharmaceuticals, biotechnology, and healthcare are expanding their research efforts, leading to an increased demand for robust data management systems. With their focus on advancing medical knowledge, developing new treatments, and improving patient outcomes, the surge in clinical trials highlights the importance of efficient data management. Clinical trials generate vast amounts of data, including patient information, treatment outcomes, and safety assessments. Effective management of this data is crucial for maintaining the integrity, accuracy, and compliance of clinical trial processes. CDMS plays a vital role in simplifying the collection, storage, and analysis of data by enhancing the effectiveness of clinical trials. As trials become more complex and regulatory standards become stricter, the need for advanced data management solutions becomes increasingly evident.
Security issues
Data collection, especially in the electronic data capture segment, raises concerns about patient privacy, potentially hindering the growth of the clinical data management system market. The increased sharing of databases among various stakeholders like CROs, research institutions, and software companies raises the risk of data breaches. While smooth access and sharing are necessary for collaboration, they also expose companies to digital security threats like data breaches, which can compromise the confidentiality, integrity, or availability of data. Such concerns about data leakage may limit the adoption of clinical data management systems by impacting market expansion.
Increasing adoption of cloud-based CDMS solutions
The clinical data management system market is experiencing a notable surge in the adoption of cloud-based solutions. Cloud technology has transformed data management and accessibility in the healthcare industry, including clinical research, due to its numerous benefits. Cloud-based CDMS platforms offer a highly scalable and flexible infrastructure, effortlessly handling the increasing volume of clinical trial data without requiring significant hardware upgrades or investments. Additionally, cloud technology enables real-time data sharing and collaboration among various stakeholders, such as researchers, investigators, and sponsors, which allows them to access and analyze data from different locations simultaneously.
The cloud-based SaaS solution segment dominated the clinical data management system market. Segmental growth in the healthcare sector is driven by technological advancements and increased adoption of cloud-based solutions. Cloud-based models offer benefits like flexibility, collaboration, and scalability, which fuel market expansion. Moreover, they eliminate the need for purchasing, maintaining, and deploying on-premises services, which reduces installation and maintenance costs and contributes to market growth.
In the clinical data management system market, the web-hosted solution segment is expected to witness significant growth over the forecast period. Web hosting services offer the hosting of various services, either shared or dedicated, for their clients. While they are commonly utilized for hosting websites, they can also be employed for hosting company email, files, games, and other types of content.
The pharma and biotech companies segment dominated the clinical data management system market. Biotechnology and pharmaceutical firms both manufacture medications, but there's a distinction in their approach. Biotech companies create medicines sourced from living organisms, whereas pharmaceutical companies typically produce medications with a chemical foundation.
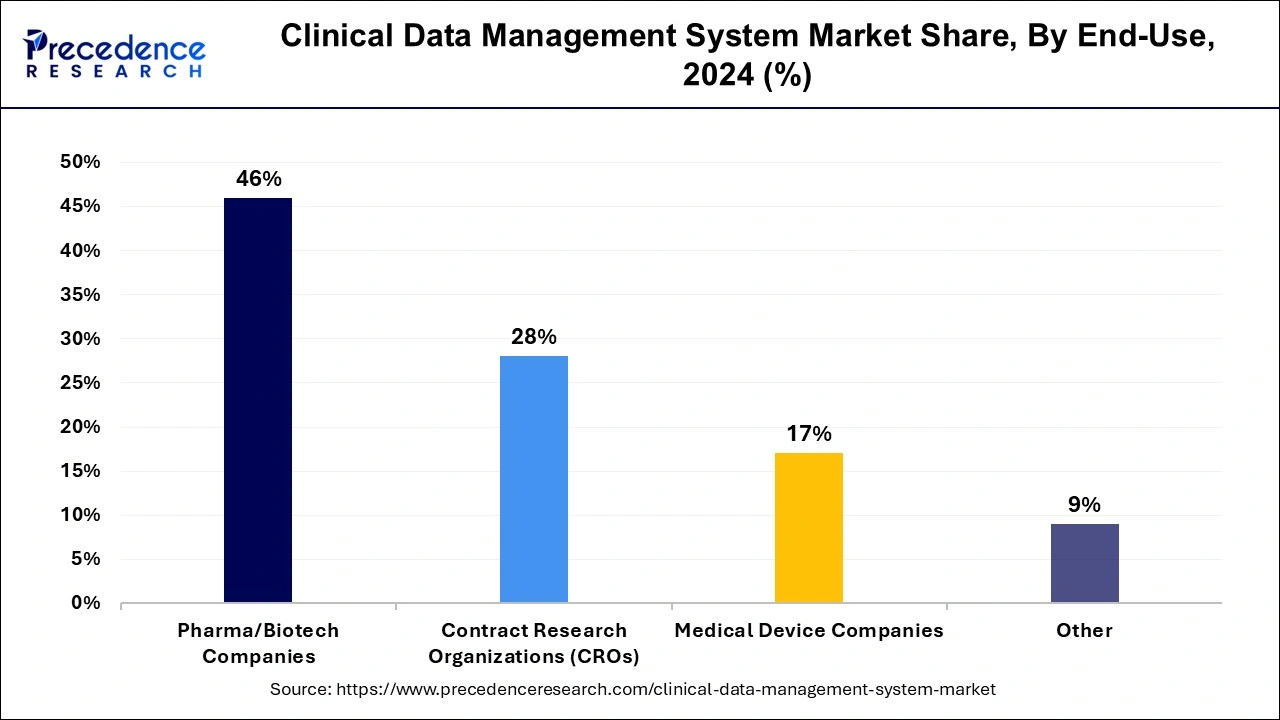
In the clinical data management system market, the contract research organization (CRO) segment is projected to show the fastest growth during the projected period. The expansion of contract research organizations (CROs) worldwide is anticipated to boost market revenue. Furthermore, the growing practice of outsourcing clinical trials to CROs, which helps lower the overall cost of drug development, is expected to drive market demand even further. Many CROs are embracing digital solutions like clinical data management systems (CDMS) and electronic data capture (EDC) to handle the increasing volume of data generated during trials effectively.
By Delivery Mode
By End-use
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
March 2025
February 2025
February 2025
January 2025