January 2025
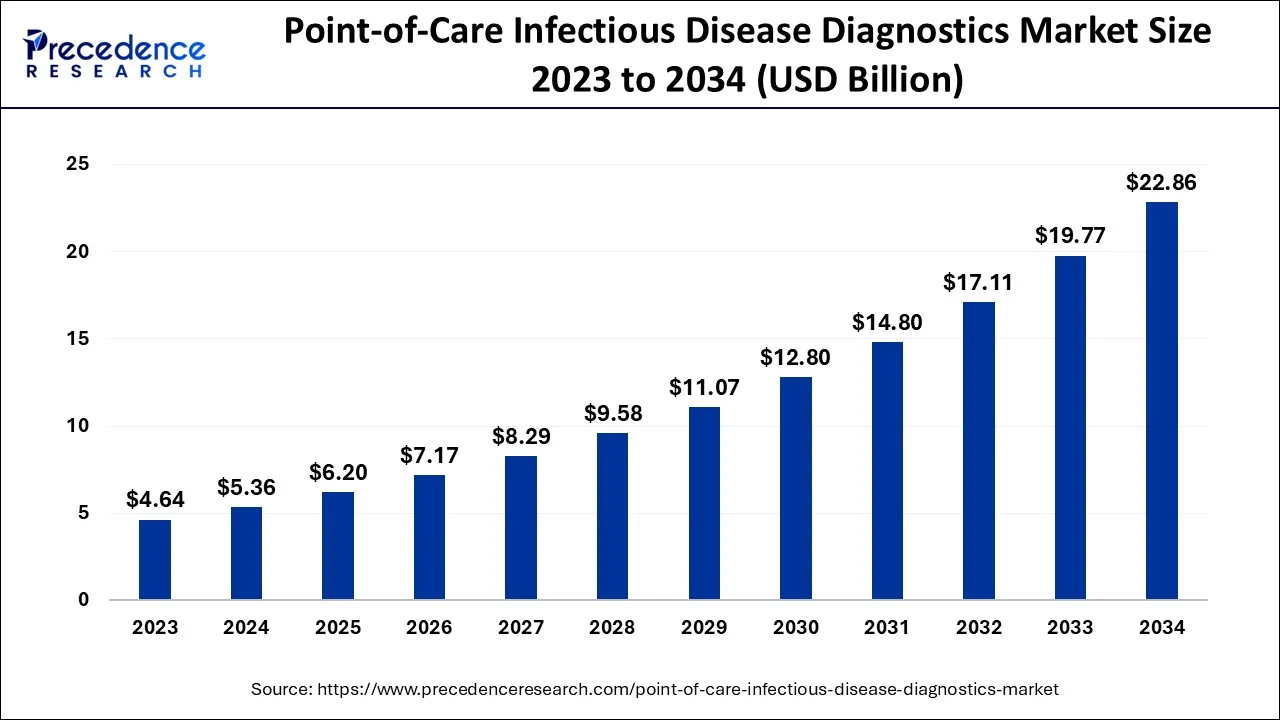
The global point-of-care infectious disease diagnostics market size is estimated at USD 5.36 billion in 2024, grew to USD 6.20 billion in 2025 and is predicted to surpass around USD 22.86 billion by 2034, expanding at a CAGR of 15.60% between 2024 and 2034.
The global point-of-care infectious disease diagnostics market size accounted for USD 5.36 billion in 2024 and is anticipated to reach around USD 22.86 billion by 2034, expanding at a CAGR of 15.60% between 2024 and 2034.
The point-of-care (POC) diagnostics market on a global level offers quick insights about patient’s health at real-time and at the place of a medical interaction. As medical testing has changed due to the demand for faster test results and the development of portable, user-friendly testing equipment. As the healthcare providers focus to invest in minimal infrastructure, the market is observed to get accelerated owing to the fact that point-of-care diagnostics for infectious diseases require low cost applications that are generally easy to manage and operate.
Point-of-care diagnostics methods will remain essential in the field of medical testing as medical care services develop to become more consumer-focused in order to improve patient outcomes. Rapid outcomes from such diagnostics methods allow immediate treatment, decrease the requirement for multiple patient visits, and aid in managing infectious disease epidemics. The rising incidence of various infectious diseases across the globe will continue to highlight the demand for point-of-care diagnostics services, it was predicted by the Centers for Disease Control and Prevention that there would be between 27 and 54 million flu cases from October 2022 to April 2023.
The demand for quick and precise diagnostic tools at the point of care has increased due to the rising incidence of infectious diseases like HIV, TB, hepatitis, and respiratory infections worldwide. Technological advancements such as molecular diagnostics, microfluidics, biosensors, and lab-on-a-chip devices have made the development of sensitive and focused point-of-care diagnostic technologies that can precisely identify infectious pathogens.
Real-time results from point-of-care diagnostics enable quick treatment initiation and lower the risk of disease transmission. Adopting such diagnostics is motivated by the demand for timely, useful information. They are instrumental in rural locations with little resources and access to centralized laboratories. These tests facilitate quick diagnosis without requiring intricate infrastructure. All these elements are observed to act as growth factors for the market.
Improving disease diagnosis and management is a significant concern for many governments and healthcare institutions. As governments and other healthcare institutions are implementing advanced diagnostic methods, the market is expected to grow at a significant rate. The trend towards home-based healthcare and telemedicine has spurred the desire for user-friendly, self-administered point-of-care tests that patients may utilize without professional assistance. Another growth factor for the market could be the rising frequency of infectious diseases among the elderly, as the world's population ages.
| Report Coverage | Details |
| Market Size in 2024 | USD 5.36 Billion |
| Market Size by 2034 | USD 22.86 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 15.60% |
| Largest Market | Asia Pacific |
| Base Year | 2023 |
| Forecast Period | 2024 To 2034 |
| Segments Covered | Technology, Application, End-user, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Rise in cases of infectious diseases
The need for quick and precise diagnostic technologies that can be used at the point of care has increased due to the rising prevalence of infectious diseases such as COVID-19, HIV, hepatitis, and tuberculosis. Point-of-care diagnostics have become more popular since many conditions require early identification for efficient management and containment. There is a greater need for detection promptly to start prompt treatment and stop the spread of infectious diseases as new ones emerge and old ones reemerge. Quick data from point-of-care diagnostic equipment enables medical personnel to act quickly and strategically when managing patients.
Rapid and precise point-of-care diagnostics assist medical professionals in prescribing focused therapies, which lowers the needless usage of broad-spectrum antibiotics. In turn, this may lessen the emergence of antibiotic resistance. The drawbacks of centralized laboratory testing have been brought to light by situations such as the COVID-19 pandemic. Point-of-care diagnostics allow decentralized testing in rural or resource-limited places, contributing to more effective disease containment. Point-of-care testing gives patients more control by giving them access to quick information about their state of health. Increased patient engagement and adherence to suggested treatment plans may result from this.
Growing demand for home testing
Self-administered point-of-care diagnostic tests that can be performed at home, such as COVID-19 or HIV, are becoming increasingly common. The ease of testing at home and the desire for discretion are the driving forces behind this development of the market. The market has responded by creating user-friendly, portable testing equipment that delivers quick findings without needing a laboratory as more individuals look for simple and fast solutions to identify ailments from the comfort of their homes. This pattern has sparked advancements in point-of-care testing technologies, opening a wider choice of accessible and cost-effective solutions for diagnosing infectious diseases without the delays brought on by conventional diagnostic techniques.
Accuracy and reliability concerns
The precision and dependability of Point-of-Care (POC) diagnostic tests might not always be on par with those of centralized lab procedures. The accuracy of results can be affected by variations in sample collection, operator abilities, and environmental factors, which may result in false positives or negatives. Diagnosing infectious diseases frequently entails complex procedures to pinpoint certain bacteria or indicators. POC tests must be easy to use, which occasionally compromises the sensitivity and specificity necessary for reliable results. It can be challenging to strike a balance between usability and clinical accuracy.
POC diagnostics are widely employed in situations with limited resources, where access to cutting-edge laboratory equipment and knowledgeable staff is constrained. The overall accuracy of diagnosis may be impacted by variances in testing methods and their interpretation. POC diagnostics frequently involve various sample types, including blood, urine, or saliva. The varying quality of multiple samples may impact the accuracy of the results. The reliability of POC tests can be affected by several variables, including sample collection, storage, and transportation.
Rising emphasis on rapid diagnostics for early treatment
POC diagnostics offer immediate results, enabling medical professionals to decide on a course of therapy quickly. This is crucial in situations like sepsis or bacterial infections where quick action can significantly improve patient outcomes. Rapid diagnostic tests assist medical professionals in promptly recognizing infectious diseases, enabling the timely start of effective therapies. This improves patient outcomes since conditions may be treated before they develop or cause problems. Healthcare professionals can counsel patients on preventive measures and lifestyle changes to slow the development of infectious diseases by identifying them early. This covers instruction on isolation, cleanliness, and vaccination. Early intervention can reduce the length of hospital stays, the requirement for intensive care, and overall healthcare expenses. It facilitates swift identification.
The lateral flow segment is expected to be dominant in the point-of-care infectious disease diagnostics market during the predicted period. Lateral flow tests are perfect for point-of-care settings when prompt judgments are essential since they produce results in only minutes. This speed makes it possible for medical professionals to launch immediate interventions and stop the spread of infectious diseases.
Lateral flow tests are portable and lightweight, making them suitable for usage in various locations, including mobile clinics, homes, and remote regions with little healthcare infrastructure. In particular, in underdeveloped areas, its portability offers greater access to diagnostics. In response to epidemics and pandemics, lateral flow testing has been crucial.
The agglutination assays segment is registered to grow faster in the point-of-care infectious disease diagnostics market during the forecast period. Agglutination assays can concurrently detect several pathogens or indicators in a single sample, resulting in a thorough diagnostic profile. This is essential for rapidly detecting co-infections or excluding particular diseases. Technology developments have produced agglutination assays that are extremely sensitive and precise. Modern tests use nanoparticles, latex beads, or magnetic particles to make agglutination responses more visible and improve diagnostic accuracy. These assays are suited for healthcare professionals with varied levels of expertise because they are simple to use and need little training. They are also flexible for use in environments with limited resources due to their simplicity.
The hospital-associated infections (HAIs) segment is expected to be dominant in the point-of-care infectious disease diagnostics market during the forecast period. Numerous reasons, such as rising hospitalization rates, an aging population, and the appearance of microorganisms resistant to antibiotics, have contributed to the rise in HAIs. Innovative POC diagnostic technologies that are highly accurate, simple to use and able to identify various infections have been created due to technological breakthroughs.
These techniques enable medical professionals to quickly pinpoint the HAI-causing substances. HAIs frequently need quick identification and treatment to stop them from spreading inside healthcare institutions. Point-of-care diagnostics deliver immediate results, enabling medical professionals to quickly implement infection control measures. There is an increasing need for quick diagnostic tools to help with early diagnosis and treatment of HAIs as patients' and healthcare professionals' awareness of the hazards associated with these infections grows.
The inflammatory disease segment is rapidly growing in the point-of-care infectious disease diagnostics market. The desire for quicker and easier diagnostic options has increased due to the rising prevalence of inflammatory disorders worldwide. To avoid complications, conditions including sepsis, rheumatoid arthritis, and inflammatory bowel disease demand quick detection and treatment. Point-of-care diagnostics provide real-time results, enabling prompt treatment choices and better patient outcomes. Point-of-care testing's affordability and simplicity have also aided in this market segment's expansion. Reduced turnaround times for test results lower the need for follow-up appointments and hospitalizations, which benefits patients and healthcare professionals.
The hospital segment is expected to be dominant in the point-of-care infectious disease diagnostics market during the forecast period. Many individuals receive medical assistance in hospitals and primary healthcare facilities, particularly for infectious disorders. They, therefore, need quick and precise diagnostic technologies to promptly recognize and treat these disorders. It often has a well-developed infrastructure and resources, such as skilled medical staff and cutting-edge laboratory facilities, allowing it to embrace various diagnostic technologies. This decreases turnaround time for results by enabling them to conduct numerous on-site tests rather than shipping samples to outside laboratories.
The diagnostic laboratories segment shows significant growth in the point-of-care infectious disease diagnostics market during the predicted period. Technology developments have produced diagnostic tools that are more precise, quick, and user-friendly. In contrast to conventional laboratory testing, these technologies enable medical personnel to promptly detect infectious diseases at the patient's bedside and shorten the turnaround time for results. Furthermore, the COVID-19 pandemic and other infectious disease outbreaks worldwide have highlighted the critical need for quick and accessible diagnostic options. Due to this demand, money has been invested in, and research has been conducted to create cutting-edge point-of-care diagnostic technologies that can promptly identify various infectious agents, from bacteria to viruses.
The home-care settings show a notable growth in the point-of-care infectious disease diagnostics market during the forecast period. Home care settings provide patients with convenience and accessibility that is unmatched. The user-friendliness of point-of-care infectious disease diagnostic tools allows patients to conduct tests without the assistance of trained medical experts. This ease of access encourages people to take charge of their health and seek prompt testing, resulting in the early identification and treatment of infectious diseases. The demand for point-of-care infectious disease diagnostics has increased due to the increased emphasis on preventative healthcare worldwide.
Better results for individuals result from routine surveillance and early illness detection, which also helps reduce disease transmission in communities. Remote patient monitoring and telehealth have gained popularity. Healthcare providers can remotely direct patients through testing procedures using point-of-care diagnostic tools.
Asia Pacific had the largest revenue share in 2023 and is expected to sustain in the point-of-care infectious disease diagnostics market throughout the predicted timeframe. Most of the world's population resides in Asia Pacific, which is also recognized to have a higher burden of infectious diseases than other areas. Contagious diseases spread because of elements including large metropolitan populations, regional healthcare disparities, and inconsistent sanitary standards. The high prevalence of diseases fuels the need for quick and convenient diagnostic tools. Asia Pacific has a wide range of geographical and climatic circumstances, which can result in infectious diseases of many kinds. This diversity calls for adaptable diagnostic approaches to address various infections and disease types. Point-of-care diagnostics provide the adaptability needed to properly handle this variability.
Numerous nations in the Asia-Pacific area are regarded as emerging economies, and their healthcare systems are expanding. Increasing attention is being paid to improving diagnostic skills, particularly at the point of care, as these economies grow, and their healthcare systems advance. This encourages using point-of-care infectious disease diagnostic tools that deliver quick, accurate results and support healthcare professionals in making prompt treatment choices.
The general public's growing knowledge of healthcare issues has increased the desire for quick and easy diagnostic solutions. Patients' increased proactivity in seeking medical care and diagnoses drives the demand for point-of-care infectious disease diagnostics. In Asia-Pacific, several nations have realized the value of early disease identification and management. Governments and health organizations have launched initiatives to increase healthcare access. All these elements are expected to continue the market’s development throughout the forecast period.
North America is expected to expand in the point-of-care infectious disease diagnostics market during the forecasted timeframe. The primary driver has been the development of quick and precise diagnostic technology. At the point of care, the identification of infectious agents could be done more quickly and accurately because of advancements in molecular diagnostics, immunoassays, and biosensors. Sending samples to centralized facilities is common in traditional lab-based procedures, which delays diagnosis. Faster results from point-of-care tests enable quicker patient treatment and infection control decision-making. Self-testing and home-based diagnostics are becoming more popular. Creating simple testing kits has made it easier for people to keep track of their health, which has helped the industry grow in North America. Electronic health records (EHRs) and point-of-care diagnostics have been integrated to improve patient data management and healthcare decision-making.
Segments Covered in the Report:
By Technology
By Application
By End-user
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
January 2025
February 2025
November 2024
October 2024