February 2025
The global AI-based clinical trials solution provider market size is accounted at USD 2.88 billion in 2025 and is forecasted to hit around USD 17.40 billion by 2034, representing a CAGR of 22.13% from 2025 to 2034. The North America market size was estimated at USD 1.04 billion in 2024 and is expanding at a CAGR of 22.23% during the forecast period. The market sizing and forecasts are revenue-based (USD Million/Billion), with 2024 as the base year.
The global AI-based clinical trials solution provider market size was calculated at USD 2.36 billion in 2024 and is predicted to reach around USD 17.40 billion by 2034, expanding at a CAGR of 22.13% from 2025 to 2034. The increased demand for personalized medicines is the key factor driving the growth of the global AI-based clinical trial solution provider market. Growing complexity in clinical trials is driving the adoption of AI-based solution providers. Additionally, the increased need for analysis of a broad amount of data is expected to continue the adoption of AI-based clinical trial solution providers.
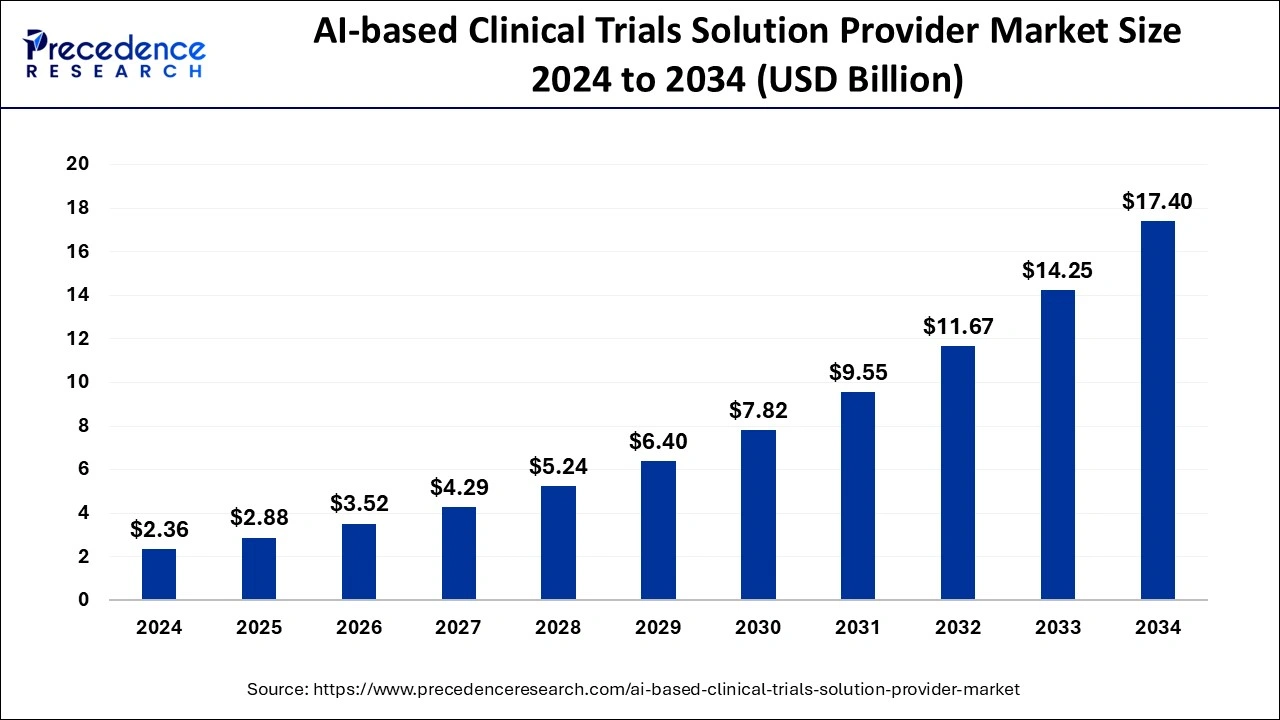
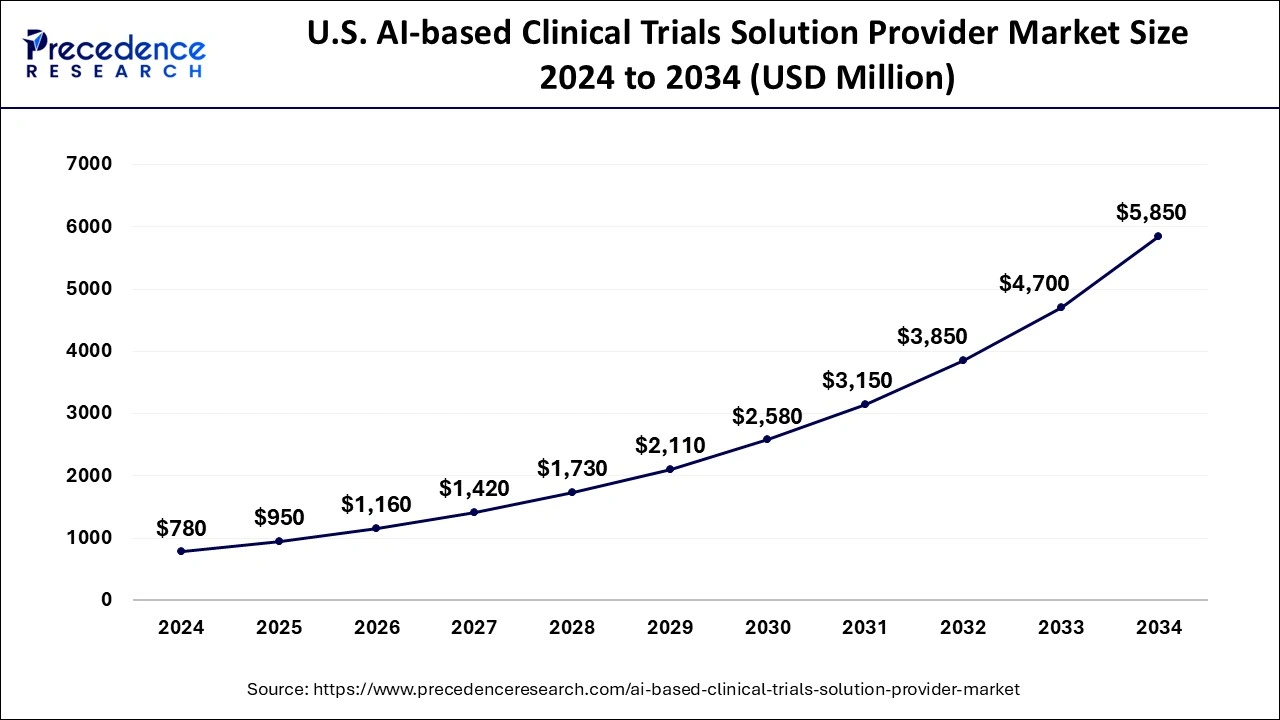
The U.S. AI-based clinical trials solution provider market size was exhibited at USD 780 million in 2024 and is projected to be worth around USD 5,850 million by 2034, growing at a CAGR of 22.32% from 2025 to 2034.
North America dominated the global AI-based clinical trials solution provider market in 2024 due to regional reach in healthcare and pharmaceutical infrastructures. Well-established healthcare in North America provides essential funding in the research and development sector and encourages the adoption of AI-based clinical trial solutions. North America is leading the market in terms of the availability of advanced healthcare infrastructure. Along with that, the ongoing pharmaceutical and biotechnology companies’ investment in R&D for the development of novel treatment solutions is encouraging the adoption of AI in clinical trials.
Early adoption of cutting-edge technologies and development is the key reason behind expanding markets in the United States. However, Canada is rapidly increasing market share due to increased regulatory and government initiatives and funding in R&D. national research council of Canada (NRC) and national institutes of health (NIH) fundings are playing crucial role support for developments of AI-based clinical trials solutions in the region. With the rise in the adoption of automation and cloud-computing technologies, the region is projected to continue dominating the market.
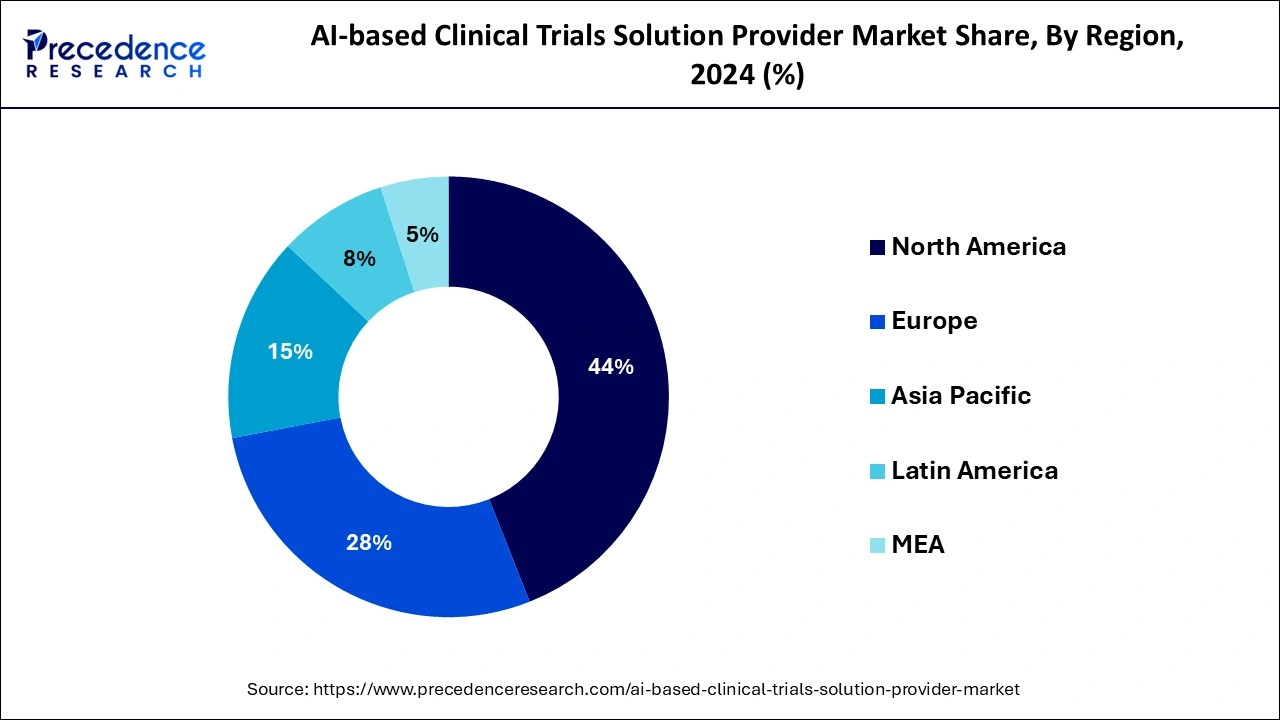
Asia Pacific will host the fastest-growing AI-based clinical trials solution provider market over the studied period due to the increased population and adoption of AI and cloud computing in the region's healthcare expenditure. The increased adoption of electronic health records (EHRs), telemedicine, and remote patient monitoring are the key trends driving the growth of the regional market. Additionally, the rising prevalence of disease and patient enrollments require vast data analytics, leading to further adoption of AI-based clinical trial solution providers.
The rising government and regulatory funding for the development of well-established healthcare, pharmaceutical, and biotechnology infrastructures are significantly fueling this growth. The adoption of advanced technologies in research and development and the gaining of funding support for the development of innovative novel treatments are highlighting the importance of AI adoption in clinical trials.
Artificial intelligence (AI) has emerged in all industries around the globe; the research and development firms are no exception. AI is allowing research, development, and implementation to witness a transforming future vision. The increased utilization of AI in medical diagnostics and remote patient monitoring systems has become the game changer in the healthcare expenditure. Similarly, the need for continuous development of innovative drugs and treatment solutions has drifted pharmaceutical and biotechnology companies’ preference toward AI.
The increased prevalence of rare and chronic diseases is leading to a need for personalized and advanced medicines, leading to ongoing cutting-edge developments. The growing pressure for the development of advanced treatment options, development timelines, and target therapies has driven AI adoption in clinical trials. The properties of AI that allow it to analyze broad amounts of data, identify targeted areas, and detect possible risks are driving the popularity of AI in clinical trials. Rapidly increased digitalization, adoption of machine learning, and cloud computing are also contributing to the market expansions.
Not only the government but also non-governmental organizations are also coming forward to invest in the adoption of AI-based clinical trial solutions in research and development. The continuously rising need for effectiveness and productivity in pharmaceutical medicines and novel treatment options is significantly driving the adoption potential of AI-based clinical trial solutions providers. The rising developments of AI-based research centers are likely to transform the market situation.
| Report Coverage | Details |
| Market Size by 2024 | USD 2.36 Billion |
| Market Size in 2025 | USD 2.88 Billion |
| Market Size in 2034 | USD 17.40 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 22.13% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Clinical Trial Phase, Therapeutic Applications, End-use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Increased focus on patient engagements
The increased focus on patient enrollment is the key driver of the AI-based clinical trials solution provider market. AI is enabled to help patient recruitment and treatment effectiveness by identifying potential candidates for clinical trials. The ability of AI to improve patient experience by improving treatment adherence is driving its popularity in clinical trials. AI helps improve patient engagement and retention via chatbots and virtual assistants.
Moreover, AI helps to generate patient-centric treatment in the AI-based clinical trials solution provider market by getting patients' feedback and preferences, which increases demand for personalized medicines. This role of AI has become essential in the trials. The real-time collection and predictive analytic properties are making it easier for researchers to understand targeted therapies and developments. The rising prevalence of disease and demands for accurate and effective treatments are leading to a need for potential patients for clinical trials; the ability to identify and comply with such patients is enhancing the popularity of AI in clinical trials.
Data privacy and security
The concerns for data privacy and security are essential for the utilization of the AI-based clinical trials solution provider market services. AI is totally reliant on machine learning algorithms, which can cause cyberattacks on patients’ sensitive data. AI-based clinical trial solution providers must comply with regulations with strict data privacy regulations. AI-based clinical trial solution providers can face cybersecurity threats and the risk of data breaches. The reliability of AI in cloud computing can lead to unauthorized access to the data. The increased cost of data security and privacy measures and regulatory complaints can impact the adoption speed of AI in clinical trials.
Increasing adoption of decentralized clinical trials
The adoption of decentralized clinical trials has increased due to patient centricity about comfort and convenience. The clinical trials form homes that make patients more comfortable and reduce the burdens of cost. The adoption of the AI-based clinical trials solution provider market allowed professionals to maintain remote patient monitoring, making it easier for decentralized clinical trials. The accessibility to enroll the patient remotely also improves patient engagement and clinical trial timelines. AI plays a crucial role in decentralized clinical trials. AI helps to provide remote data collection for patient monitoring.
Additionally, the ability of AI to provide predictive analytics for possible risks is allowing for proactive measurements. The AI-enabled chatbots and virtual assistants also help deliver support and improve patient retention. The ability of AI to provide streamlined data processing, minimize errors, enhance data quality, and automate data management is helping with the easy adoption of decentralized clinical trials. The benefit of AI in complying with decentralized clinical trials is that it holds potential for the AI-based clinical trials solution provider market.
The phase II segment accounted for the biggest share of the AI-based clinical trials solution provider market in 2024. The segment growth is attributed to the ability of AI to provide accuracy and efficient data analysis. Phase II clinical trials require the efficacy and safety of novel treatment solutions. The need for accurate dose-ranging studies and candidate selections and the detection of possible side effects are the other major factors driving the expansion of the segment. Advanced technologies are allowing AI-powered natural language processing to analyze vast amounts of clinical trial data, making it essential for phase II trials. The ability of AI to reduce time, cost, and side effects helps to improve trial efficiency and is the major reason for the adoption of AI in phase II clinical trials.
The Phase I segment is expected to witness significant growth in the AI-based clinical trials solution provider market over the forecast period due to increased adoption of AI in Phase I trials for early-stage decision-making and the execution of first-in-human (FIH) studies to reduce risk, side effects, expenses, and time consumption. The need for accurate management and future outlook in Phase I clinical trials is the key factor that has enhanced the adoption rate of AI. The collected data can be further improved with the help of AI, which helps to reduce error risks and inconsistencies. Additionally, with growing pressure for the development of novel drugs or treatment solutions, pharmaceutical and research companies are attracted to the adoption of AI to satisfy development timelines.
The oncology segment dominated the AI-based clinical trials solution provider market in 2024. The increased prevalence of cancers globally is requiring personalized medicines. The ability of AI to identify tailored treatment options for individual patients is the major reason behind the rapidly increased utilization of AI in oncology treatment research and developments. The need for cutting-edge tools to deal with the complexity of cancer biology during clinical trials and identify potential therapeutic targeted areas is significantly driving the shift to the adoption of AI-powered tools in clinical trials for cancers. Advanced AI technologies like ML, NL, and deep learning are enabled to deliver accurate, efficient, targeted, and precise treatment solutions for oncology patients.
However, the cardiovascular disease (CVD) segment will grow considerably in the AI-based clinical trials solution provider market during the forecast period due to the growing utilization of AI-powered platforms to analyze complex CVD data and detect potential therapeutic targets. The complexity and heterogeneity of CVD have made it essential for AI utilization to improve treatment accuracy and efficiency. The ability of AI to analyze vast data sources from imaging and HER and deliver real-time evidence to improve the effectiveness of novel treatments and identify possible risks are driving the adoption of AI in clinical trials for cardiovascular disease.
The pharmaceutical companies segment led the global AI-based clinical trials solution provider market in 2024 due to increased research and developments for novel treatment options and rising pharmaceutical investment in the clinical trials of new studies. The ongoing research and clinical trials need AI to analyze large amounts of data and identify potential patterns. The AI's ability to analyze and optimize complex data is the prior reason for the increased adoption of AI by pharmaceutical companies. Additionally, rising research and developments of novel biomarkers are also contributing to segment expansion. With the growing prevalence of rare diseases, pressure for development timelines, rising demands for personalized medicines, and the need for remote healthcare facilities, pharmaceutical companies are anticipating that they will rapidly adopt AI.

By Clinical Trial Phase
By Therapeutic Applications
By End-use
By Geography
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
February 2025
November 2024
October 2024
January 2025